
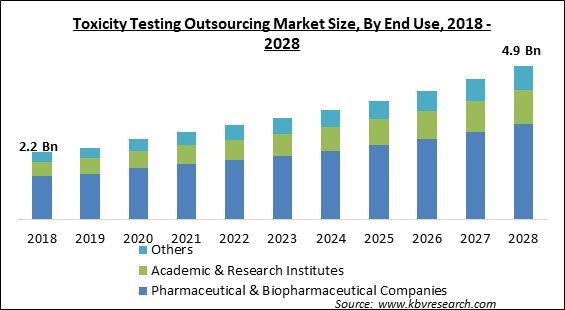
The Global Toxicity Testing Outsourcing Market size is expected to reach $4.9 billion by 2028, rising at a market growth of 8.4% CAGR during the forecast period.
Toxicity testing is the process of assessing the extent to which an ingredient of interest adversely affects an organism's regular biological functions, given a specific exposure time, exposure route, and substance concentration. It is also known as toxicology testing or safety assessment. For a specific drug, exposure environment, route, time, or for a specific target organism, or for a certain developmental stage of interest, toxicological testing is frequently carried out by researchers.
Preclinical testing for a chemical destined for human exposure frequently includes toxicology testing. To establish safe exposure dosages in model organisms, various stages of in silico, in vitro, and in vivo research are carried out. The next stage of research entails first-in-man studies for human toxicological testing, if necessary.
The pharmaceutical business, biotechnology firms, contract research groups, or environmental scientists may all carry out toxicology testing. Testing for toxicity looks at the chemical components of completed items such as pesticides, drugs, cosmetics, food additives like packing materials, artificial sweeteners, and air fresheners. A variety of techniques are used to evaluate the compounds, including cutaneous application, respiration, oral intake, injection, and aquatic environments.
They can be rubbed on the skin or eyes, injected intramuscularly, intravenously, or subcutaneously or inhaled by covering the animals with a mask or putting them in a respiration chamber. They can also be given orally by mixing them into the animals' food or inserting a tube into their stomachs. Testing for toxicity can also be done on substances that need to be discarded, like silt that needs to be dumped in a marine environment.
Companies have, however, adopted a number of techniques, such as varying shifts, downsizing staff, and working remotely, to lessen or eliminate these issues. Numerous businesses provided innovative testing and solutions to aid the government's attempts to halt the COVID-19 outbreak. These companies were able to lessen the impact COVID-19 has on their daily operations. The reduced working of these laboratories necessitated the need for outsourcing testing and research. Therefore, COVID-19 propelled the trend of outsourcing and as such had a positive impact on the toxicity testing outsourcing market.
For the sale and importation of some consumer goods, genetically modified eatables, medical devices, vaccines, pharmaceuticals, and industrial chemicals, and pesticides, government rules in a number of nations demand genotoxicity testing on animals. Millions of animals are used every year for drug discovery, including monkeys, birds, hamsters, guinea pigs, frogs, cats, and mice. As a result, numerous animal ethical independent committees have been established in numerous countries all over the world.
Strategies to establish or predict safety and effectiveness in humans prior to drugs entering clinical trials could significantly lower the failure rate of novel therapeutics. More focus has been placed on in vitro drug discovery and personalized treatments. Additionally, there is a growing demand for humanistic animal models, which will most likely open up a variety of opportunities for rivals in gene toxicology research in the near future. Predetermining the toxicity of intended drugs could help in mitigating the negative effects.
In vitro toxicity assessment methodologies are being used more often across a range of sectors. Regulatory agencies may substitute validated alternative testing for in vivo tests. Several nations have expressed a reluctance to adopt in vitro testing instead of in vivo procedures, even though the assay has proven its greater scientific relevance. In the United States, a number of regulatory agencies continue to use data derived from animal testing. Many organizations use in vivo approaches to evaluate novel toxicological test methods.
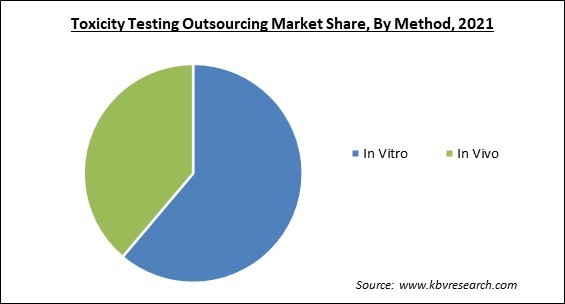
Based on method, the toxicity testing outsourcing market is categorized into in vitro and in vivo. The in vivo segment garnered a significant revenue share in the toxicity resting outsourcing market in 2021. An essential component of medical research generally is in vivo testing, particularly in clinical trials. Studies conducted in living organisms (in vivo) offer useful knowledge about a substance's effects or the progression of a disease.
On the basis of GLP type, the toxicity testing outsourcing market is bifurcated into GLP and non-GLP. The GLP (Good Laboratory Practices) segment procured the largest revenue share in the toxicity testing outsourcing market in 2021. For safe exposure to people, clinical studies such as safety pharmacology, genotoxicity, and repeated dosage toxicity must be conducted in accordance with GLP guidelines. These research projects must be finished before submitting an IND application.
Based on end-use, the toxicity testing outsourcing market is divided into pharmaceutical & biopharmaceutical companies, academic & research institutes, and others. The pharmaceutical & biopharmaceutical companies segment recorded the maximum revenue share in the toxicity testing outsourcing market in 2021. This is because small and midsize pharmaceutical businesses, that lack experience in the preclinical phase of toxicity testing of drug development, are increasingly outsourcing end-to-end services.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 2.8 Billion |
| Market size forecast in 2028 | USD 4.9 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 8.4% from 2022 to 2028 |
| Number of Pages | 188 |
| Number of Tables | 330 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | GLP, Method, End-use, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
On the basis of region, the toxicity testing outsourcing market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North American region witnessed the highest revenue share in the toxicity testing outsourcing market in 2021. The availability of enough infrastructure to enable the development and growth of drug discovery technologies, an increase in medical spending, and an intense emphasis on drug development by governmental agencies are all contributing factors.
Free Valuable Insights: Global Toxicity Testing Outsourcing Market size to reach USD 4.9 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Thermo Fisher Scientific, Inc., Eurofins Scientific Group, WuXi AppTec Co., Ltd., SGS S.A., Intertek Group PLC, Catalent, Inc., Laboratory Corporation of America Holdings, Charles River Laboratories International, Inc., ICON PLC, and Medpace Holdings, Inc.
By End Use
By Method
By GLP
By Geography
The global Toxicity Testing Outsourcing Market size is expected to reach $4.9 billion by 2028.
Acceptance Of Outsourcing Toxicity Tests Among Animal Care Societies are driving the market in coming years, however, Scarcity Of Skilled Professionals And Reluctance Of Regulatory Bodies restraints the growth of the market.
Thermo Fisher Scientific, Inc., Eurofins Scientific Group, WuXi AppTec Co., Ltd., SGS S.A., Intertek Group PLC, Catalent, Inc., Laboratory Corporation of America Holdings, Charles River Laboratories International, Inc., ICON PLC, and Medpace Holdings, Inc.
The expected CAGR of the Toxicity Testing Outsourcing Market is 8.4% from 2022 to 2028.
The In Vitro segment acquired maximum revenue share in the Global Toxicity Testing Outsourcing Market by Method in 2021 thereby, achieving a market value of $2.9 billion by 2028.
The North America market dominated the Global Toxicity Testing Outsourcing Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $1.2 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
