
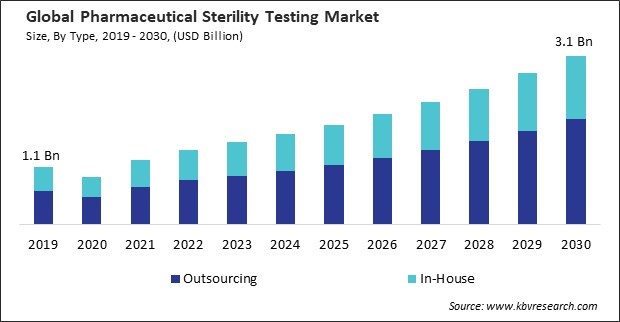
The Global Pharmaceutical Sterility Testing Market size is expected to reach $3.1 billion by 2030, rising at a market growth of 10.7% CAGR during the forecast period.
Advances in medical technology have led to the development of increasingly complex medical devices. Consequently, the medical device companies by end-use would generate approximately 21.6% share in the market by 2030. The complexity of these devices may require more sophisticated sterility testing methodologies to ensure their safety and efficacy. This complexity can drive the demand for specialized sterility testing services tailored to the unique characteristics of medical devices.
Health authorities, including the FDA and EMA, maintain a global pharmaceutical quality and safety perspective. The regulatory frameworks established by these bodies set the benchmark for sterility testing requirements. As a result, pharmaceutical manufacturers must align their processes with these standards to navigate the complex landscape of international regulatory compliance. Hence, owing to these factors, there will be increased demand for pharmaceutical sterility testing.
Additionally, Patient safety is a top priority in healthcare, and any compromise in the sterility of pharmaceutical products can pose serious risks to patients. Infections resulting from contaminated drugs or medical devices can lead to adverse health outcomes and increased healthcare costs. Thus, these factors will fuel the demand for pharmaceuticals in the upcoming years.
However, Regulatory bodies, such as the United States Pharmacopeia (USP) and the European Pharmacopoeia (EP), enforce stringent guidelines for sterility testing to ensure the safety and efficacy of pharmaceutical products. Hence, these factors can lead to reduced demand in the pharmaceutical market.
Furthermore, the increased demand for these products emphasized the need for rigorous sterility testing procedures to guarantee their safety and effectiveness. The pandemic necessitated expedited timelines for developing, testing, and approving pharmaceutical products, including vaccines. Hence, the COVID-19 pandemic positively impacted the pharmaceutical market.
 Drivers
Drivers  Restraints
Restraints  Opportunities
Opportunities  Challenges
Challenges Based on type, the market is segmented into in-house and outsourcing. In 2022, the outsourcing segment held 59% revenue share in the market. Outsourcing sterility testing offers cost-efficiency benefits. Establishing and maintaining an in-house sterility testing facility involves substantial capital investment, ongoing operational costs, and the need for skilled personnel. Hence, these factors will help in the expansion of the segment.
On the basis of product, the market is divided into kits & reagents, instruments, and services. In 2022, the instruments segment witnessed a 25% revenue share in the market. Technological advances have led to the development of sophisticated instruments that offer improved capabilities for sterility testing. Automated systems, robotics, and advanced analytical instruments contribute to the overall efficiency of testing processes, allowing for faster and more accurate detection of microbial contaminants. Therefore, these factors can drive the expansion of the segment.
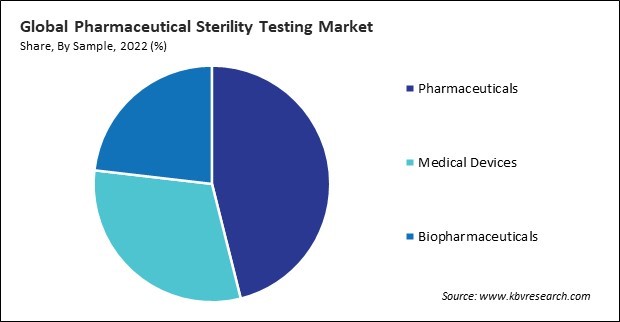
Based on sample, the market is segmented into pharmaceuticals, medical devices, and biopharmaceuticals. The pharmaceuticals segment held the 46% revenue share in the market in 2022. Many pharmaceutical companies increasingly outsource manufacturing and testing activities to contract service providers. This trend can contribute to the growth of the market, with contract testing laboratories playing a key role in providing sterility testing services. Therefore, these factors can lead to enhanced demand in the segment.
On the basis of end-use, the market is divided into compounding pharmacies, medical device companies, pharmaceutical companies, and others. The compounding pharmacies segment recorded an 11% revenue share in the market in 2022. Compounding pharmacies customize medications to meet the unique needs of individual patients. They are crucial in providing tailored solutions for patients requiring specific formulations, dosages, or delivery methods not readily available in commercially manufactured medications. Hence, these factors will pose lucrative growth prospects for the segment.
Based on test type, the market is divided into sterility testing, bioburden Testing, and bacterial endotoxin testing. In 2022, the bioburden testing segment recorded 42.5% revenue share in the market. Bioburden testing plays a key role in meeting regulatory requirements and ensuring compliance with quality standards. Regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have strict guidelines for controlling microbial contamination in pharmaceutical products. Thus, these factors can assist in the expansion of the segment.
Free Valuable Insights: Global Pharmaceutical Sterility Testing Market size to reach USD 3.1 Billion by 2030
By region, the market is segmented into North America, Europe, Asia Pacific, and LAMEA. In 2022, the Europe segment acquired a 26.17% revenue share in the market. Europe has a well-established pharmaceutical industry with a diverse range of products. The continuous growth of the pharmaceutical sector, including the development of new drugs and biologics, contributes to the demand for sterility testing to ensure product safety and efficacy. Therefore, these factors will pose lucrative growth prospects for the segment.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 1.4 Billion |
| Market size forecast in 2030 | USD 3.1 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 10.7% from 2023 to 2030 |
| Number of Pages | 314 |
| Number of Tables | 530 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Porter’s 5 Forces Analysis, Company Profiling, Companies Strategic Developments, SWOT Analysis, Winning Imperatives |
| Segments covered | Type, Product Type, Sample, Test Type, End-use, Region |
| Country scope |
|
| Companies Included | Steris PLC, Charles River Laboratories International, Inc., Thermo Fisher Scientific, Inc., SGS S.A., Sartorius AG , Sotera Health Company, Pacific Biolabs, Inc., Laboratory Corporation of America Holdings, Almac Group, Pace Analytical Services, LLC |
By Type
By Product Type
By Sample
By End Use
By Test Type
By Geography
This Market size is expected to reach $3.1 billion by 2030.
Stringent Regulatory Standards and Compliance Requirements are driving the Market in coming years, however, Time-Consuming Testing Processes and Time-To-Market Pressures restraints the growth of the Market.
Steris PLC, Charles River Laboratories International, Inc., Thermo Fisher Scientific, Inc., SGS S.A., Sartorius AG , Sotera Health Company, Pacific Biolabs, Inc., Laboratory Corporation of America Holdings, Almac Group, Pace Analytical Services, LLC
The expected CAGR of this Market is 10.7% from 2023 to 2030.
The Kits & Reagents segment is leading the Market by Product Type in 2022 there by, achieving a market value of $1.8 Billion by 2030.
The North America region dominated the Market by Region in 2022, and would continue to be a dominant market till 2030; there by, achieving a market value of $1.4 Billion by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
