According to a new report, published by KBV research, The Global Pharmaceutical Sterility Testing Market size is expected to reach $3.1 billion by 2030, rising at a market growth of 10.7% CAGR during the forecast period.
The development of advanced microbial detection systems, including real-time polymerase chain reaction (PCR) and next-generation sequencing, enhances the sensitivity and specificity of sterility testing. These technologies enable rapid and precise identification of microorganisms. Real-time PCR offers heightened sensitivity and specificity compared to traditional culture-based methods. It can detect low levels of microbial contamination that may go undetected in culture-based approaches. The specificity is achieved by designing primers that selectively target the DNA or RNA of particular microorganisms, providing a more accurate and reliable identification.
The In-House segment is experiencing a CAGR of 9.5% during (2023 - 2030). In-house sterility testing allows pharmaceutical companies to integrate quality control measures seamlessly into their manufacturing processes. By having sterility testing facilities within their premises, companies can conduct testing at various stages of production, ensuring real-time monitoring and immediate corrective actions if needed. Thus, these factors will boost the demand in the segment.
The Kits & Reagents segment is leading the Global Pharmaceutical Sterility Testing Market by Product Type in 2022 thereby, achieving a market value of $1.8 Billion by 2030. Kits and reagents provide a convenient and standardized approach to sterility testing. These ready-to-use solutions often come with pre-measured and pre-qualified components, ensuring consistency and reliability in testing processes. The standardization offered by kits and reagents is particularly valuable in maintaining quality across different batches and manufacturing facilities. Therefore, these factors can help in the growth of the segment.
The Medical Devices segment is anticipated to have a CAGR of 10.9% during (2023 - 2030). The rising significance of medical devices has driven advancements in sterility testing technologies. Laboratories and pharmaceutical companies are investing in state-of-the-art equipment and techniques to ensure the accurate and efficient testing of medical devices. This may include adopting rapid sterility testing methods and advanced microbial detection systems. Thus, the segment will expand rapidly in the upcoming years.
The Pharmaceutical Companies segment is registering a strong potential in the Global Pharmaceutical Sterility Testing Market by End-use in 2022 thereby, achieving a market value of $1.9 billion by 2030. Pharmaceutical companies utilize sterility testing for quality assurance, regulatory compliance, and batch release of sterile products. It validates aseptic processes, aids in environmental monitoring, and supports research and development. By mitigating contamination risks, it ensures product safety and upholds industry standards, safeguarding both patients and reputation.
The Sterility Testing segment is poised to have a CAGR of 10.9% during (2023 - 2030). There has been a growing interest in rapid sterility testing methods that provide results faster than traditional culture-based methods. Technologies such as real-time polymerase chain reaction (PCR), nucleic acid amplification, and other molecular-based techniques may be gaining prominence. Thus, these factors will fuel the demand in the segment.
Full Report: https://www.kbvresearch.com/pharmaceutical-sterility-testing-market/
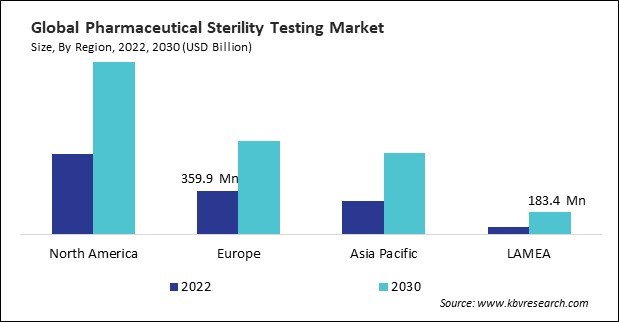
The North America region dominated the Global Pharmaceutical Sterility Testing Market by Region in 2022, and would continue to be a dominant market till 2030; thereby, achieving a market value of $1.4 Billion by 2030. The Europe region is experiencing a CAGR of 10.3% during (2023 - 2030). Additionally, The Asia Pacific region would exhibit a CAGR of 11.8% during (2023 - 2030).
By Type
By Product Type
By Sample
By End Use
By Test Type
By Geography
 Unique Offerings
Unique Offerings