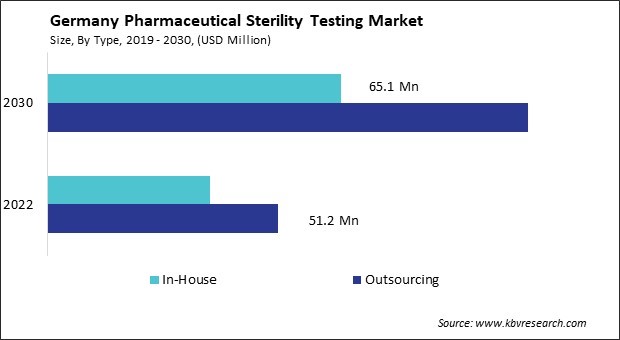
The Germany Pharmaceutical Sterility Testing Market size is expected to reach $171.5 Million by 2030, rising at a market growth of 9% CAGR during the forecast period.
Germany's pharmaceutical sterility testing market has experienced significant growth in recent years, driven by increasing regulations, technological advancements, and the rising demand for safe and effective pharmaceutical products. Sterility testing is a critical step in pharmaceutical manufacturing to ensure that drugs are free from microbial contamination, which pose serious health risks to patients. As one of the leading pharmaceutical industries in Europe, Germany has a robust regulatory framework and stringent quality standards, making it a key industry for sterility testing products and services.
According to Germany Trade & Invest, Germany stands as a prominent global hub for pharmaceutical production, boasting a substantial increase in production volume, reaching EUR 34.6 billion in 2021 with a remarkable 6.9 % year-on-year growth. The subsequent year, 2022, witnessed a notable surge in pharmaceutical industry sales within Germany, marking 5.4 % and reaching EUR 56.5 billion at ex-manufacturer prices. As demand for pharmaceuticals rises, ensuring product safety and efficacy becomes paramount. Germany's pharmaceutical sterility testing market is poised for significant growth as stakeholders prioritize stringent quality control measures to maintain the industry's reputation for delivering safe and effective medications.
One of the key factors driving the growth of the pharmaceutical sterility testing market in Germany is the increasing emphasis on regulatory compliance. Regulatory authorities such as the European Medicines Agency (EMA) and the German Federal Institute for Drugs and Medical Devices (BfArM) have established stringent guidelines governing sterility testing practices. Pharmaceutical companies in Germany demonstrate compliance with these regulations to obtain product approvals and maintain industry access, driving the demand for robust sterility testing solutions.
The COVID-19 pandemic has significantly impacted the pharmaceutical sterility testing market in Germany. The outbreak prompted an unprecedented demand for pharmaceutical products to combat the virus, including vaccines, antiviral drugs, and medical devices. In response to the pandemic, regulatory agencies introduced expedited approval pathways and flexibilities to facilitate the rapid development and deployment of COVID-19-related products.
The pharmaceutical sterility testing market in Germany has witnessed a significant trend towards outsourcing testing services. One of the primary drivers behind the increasing outsourcing of sterility testing services is the growing complexity and stringent regulatory requirements governing pharmaceutical manufacturing. With regulatory bodies such as the European Medicines Agency (EMA) and the German Federal Institute for Drugs and Medical Devices (BfArM) imposing rigorous standards, pharmaceutical companies are under immense pressure to comply with these requirements while maintaining efficiency and cost-effectiveness.
Outsourcing sterility testing allows pharmaceutical companies in Germany to leverage the expertise and specialized facilities of contract testing laboratories. By outsourcing, pharmaceutical companies in Germany access a broader range of testing capabilities without investing in expensive equipment and infrastructure. Moreover, outsourcing offers pharmaceutical companies greater flexibility and scalability in their operations. They adapt to fluctuating testing demands more efficiently by utilizing the services of contract laboratories, thereby avoiding the need for significant capital investments in in-house testing facilities.
Furthermore, outsourcing mitigates the risk of contamination and cross-contamination in pharmaceutical manufacturing processes. Contract laboratories adhere to strict protocols for sample handling and testing procedures, reducing the likelihood of errors and ensuring the integrity of test results. Therefore, the trend towards outsourcing sterility testing in Germany provides pharmaceutical companies with enhanced compliance, efficiency, and risk mitigation in line with stringent regulatory requirements.
In Germany, the pharmaceutical sterility testing market is witnessing a notable shift towards the increasing popularity of direct inoculation methods. Direct inoculation, or direct transfer or membrane filtration, involves introducing a sample into the test environment without prior incubation in a growth medium. One key advantage is the potential for reduced time to results. Direct inoculation methods typically require shorter incubation periods than traditional methods, enabling faster detection of microbial contamination.
Additionally, direct inoculation methods offer enhanced sensitivity and specificity in detecting low levels of microbial contamination. By eliminating the need for sample dilution, these methods minimize the risk of false-negative results, ensuring greater confidence in the sterility assessment of pharmaceutical products. Direct inoculation methods are increasingly recognized and accepted by authorities such as the European Pharmacopoeia. This recognition reflects the growing confidence in the reliability and validity of these techniques for pharmaceutical sterility testing.
Moreover, adopting automation and robotics in laboratory workflows has further facilitated the implementation of direct inoculation methods. Automated systems not only streamline the testing process but also reduce the potential for human error, enhancing test results' overall quality and consistency. Thus, the rising popularity of direct inoculation methods in Germany's pharmaceutical sterility testing market is driven by their efficiency, sensitivity, and regulatory acceptance.
Germany stands as a pivotal hub in the global pharmaceutical industry, not only for its robust manufacturing capabilities but also for its stringent adherence to quality and safety standards, of which sterility testing is crucial. Sartorius AG is one of the most prominent companies in this sector, headquartered in Göttingen, Germany. It is renowned for its comprehensive range of products and services catering to biopharmaceutical manufacturing and laboratory applications, including advanced sterility testing solutions. Sartorius offers a variety of filtration and separation technologies, microbiological testing kits, and services that ensure compliance with international sterility standards. Their innovative approaches and commitment to quality have made them a leading choice for pharmaceutical companies worldwide.
Another significant contributor to the German pharmaceutical sterility testing market is Robert Bosch GmbH, through its subsidiary Bosch Packaging Technology, now known as Syntegon Technology. While Bosch is traditionally known for its engineering and technology solutions, its foray into pharmaceutical packaging machinery includes offerings that ensure the sterility of pharmaceutical products. This includes advanced aseptic filling and sealing systems, which play a critical role in maintaining product sterility up to the point of use.
In addition to these global giants, Germany's pharmaceutical sterility testing market is supported by specialized firms like Mikrolab GmbH. Based in Germany, Mikrolab offers various services tailored to the pharmaceutical industry, including microbiological and sterility testing according to international pharmacopeia standards. Their focus on personalized service and technical excellence makes them a preferred partner for pharmaceutical companies looking for reliable sterility testing solutions.
Germany is also home to Vetter Pharma, a leading contract development and manufacturing organization (CDMO) that specializes in the aseptic filling of syringes, cartridges, and vials. While not a sterility testing company per se, Vetter Pharma's state-of-the-art facilities and stringent quality control measures include comprehensive sterility testing to ensure the safety and efficacy of the injectable medications they produce. Their expertise highlights the integrated approach to sterility in the pharmaceutical production process in Germany.
Hence, the collaborative efforts between these companies and regulatory bodies ensure continuous improvement in sterility testing methods, incorporating the latest scientific advancements and technological innovations. This dynamic environment supports the German pharmaceutical industry and has a far-reaching impact on global public health by ensuring the production of sterile and safe pharmaceutical products.
By Type
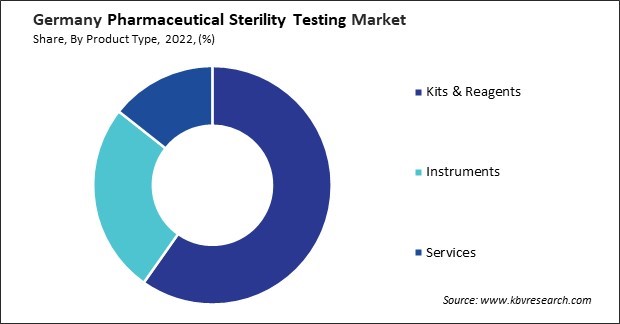
By Product Type
By Sample
By End Use
By Test Type
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

