
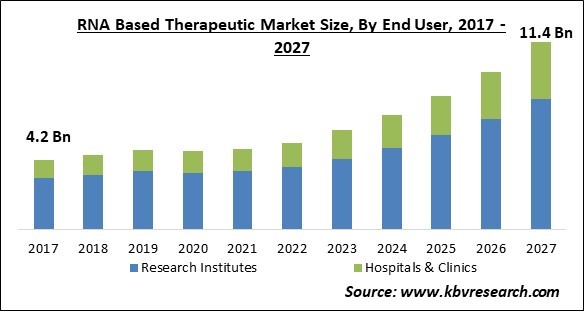
The Global RNA Based Therapeutic Market size is expected to reach $11.4 billion by 2027, rising at a market growth of 15.1 % CAGR during the forecast period.
RNA-based treatments have received a lot of popularity in recent years because of their potential to cure a wide range of chronic diseases like cancer, diabetes, AIDS, tuberculosis, and certain cardiovascular ailments, as well as uncommon and hereditary abnormalities. RNA-based treatments have been investigated as a viable treatment option for difficult-to-treat disorders, despite being in the clinical development phase.
The therapies are being developed using promising technologies such as RNA interference (RNAi), antisense technology, and SMaRT technology. RNAi and antisense technology are gaining popularity in the research business because they give the base sequence for developing RNA therapeutics. The potential for RNA to be employed for therapeutic purposes has been explored, and specific problems have arisen during drug discovery and the implementation of RNA therapies.
Coronavirus (COVID-19) was discovered in Wuhan, Hubei Province, China, in December 2019. The disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, which can be rapidly spread between people. Moreover, this virus causes a variety of symptoms in individuals, ranging from minor to severe fever, dry cough, and exhaustion. These are some of the most prevalent symptoms. Breathing difficulties or shortness of breath, chest pain or pressure, and lack of speech or movement are all dangerous signs.
In addition, among the older population, the virus has a high risk of causing death. The World Health Organization (WHO) has declared COVID-19 a pandemic as of March 11, 2020. Furthermore, only a few vaccines have been licensed for COVID-19 in the event of an emergency.
Demand is fueled by the presence of a large number of patients with infectious and chronic diseases. The desire for cures for infectious diseases and cancer is projected to influence the growth of the global RNA-based therapies and vaccine market. Moreover, according to the data shared by the WHO, Cancer is the second biggest cause of death worldwide, accounting for 9.6 million fatalities in 2018, or one in every six deaths. Men's cancers include lung, colorectal, stomach, prostate, and liver cancer, whereas women's cancers include breast, lung, cervical, colorectal, and thyroid cancer.In addition, the patient pool is large, which is expected to be another growth factor for the worldwide RNA-based therapies and vaccine market in the approaching years.
A large number of people are currently undergoing various drug courses as a result of a broad range of disorders. The remedies are made with a variety of salts and other ingredients and are intended for broad use. Regular drugs are supplied to clients in accordance with their treatment needs. Acids and salts, as well as other ingredients found in generic treatments, are not suitable for everyone. Due to RNA-based therapeutic, the practitioners are allowed to provide patients with personalized medicines based on their bodies' needs.
Because mRNA is such a massive and hefty molecule (105 106 Da), successfully translating it into pharmaceuticals poses numerous problems. Additionally, mRNA is unstable and susceptible to nuclease degradation, as well as activating the immune system. In addition, because mRNA has a large negative charge density, it is less permeable across cell membranes. Because of these factors, mRNA is easily destroyed in the absence of a suitable delivery method, with a half-life of only around 7 hours. Despite the fact that chemical changes have helped to overcome some obstacles, mRNA delivery remains a challenge. Microinjection, RNA patches (mRNA loaded in a dissolving micro-needle), gene gun, protamine condensation, RNA adjuvants, and encapsulating mRNA in nanoparticles with lipids are some of the approaches that have been studied to increase mRNA delivery.
Based on Type, the market is segmented into RNA Antisense and RNA Interference (RNAi). The RNA antisense segment dominated the RNA-based therapeutics market by acquiring the largest revenue share. The growth of this segment is attributed to its property of treating multiple diseases. Antisense RNA is utilized in cancer treatment, metastasis inhibition, and antisense sequestration vectors.
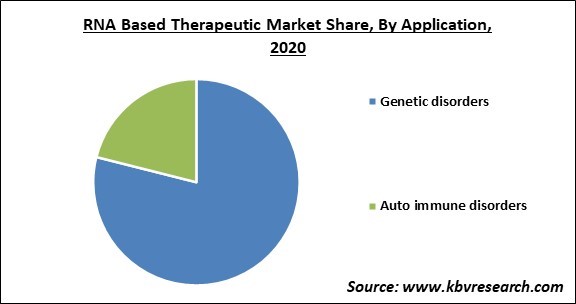
Based on Application, the market is segmented into Genetic disorders and Auto immune disorders. The autoimmune disorders segment accounted for significant revenue share. A disorder in which the immune system accidentally targets the body itself is known as autoimmune disease. Normally, the immune system protects us against pathogens such as bacteria and viruses.
Based on End User, the market is segmented into Research Institutes and Hospitals & Clinics. The research institutes segment dominated the market by accounting for the largest revenue share. Due to the increasing number of collaborations between research organizations and academic research institutions or cooperating with government agencies in order to bear the high cost of research, the research institutes segment observed an accelerated growth.
| Report Attribute | Details |
|---|---|
| Market size value in 2020 | USD 4.7 Billion |
| Market size forecast in 2027 | USD 11.4 Billion |
| Base Year | 2020 |
| Historical Period | 2017 to 2019 |
| Forecast Period | 2021 to 2027 |
| Revenue Growth Rate | CAGR of 15.1% from 2021 to 2027 |
| Number of Pages | 182 |
| Number of Tables | 323 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Type, Application, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. North America dominated the market by accounting for the largest revenue share. Research efforts for RNA-based treatments are expected to increase in North America as a result of a strong infrastructure for developmental research, the availability of substantial research funds, and increased government initiatives toward RNA-based medicines.
Free Valuable Insights: Global RNA Based Therapeutic Market size to reach USD 11.4 Billion by 2027
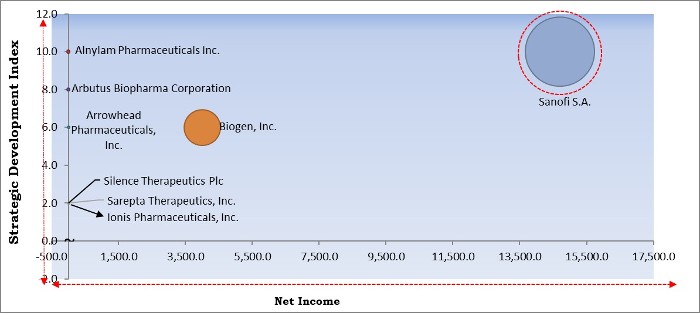
The major strategies followed by the market participants are Partnerships. Based on the Analysis presented in the Cardinal matrix; Sanofi S.A. is the major forerunner in the RNA Based Therapeutic Market. Companies such as Alnylam Pharmaceuticals Inc., Biogen, Inc., Arbutus Biopharma Corporation are some of the key innovators in the Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Arbutus Biopharma Corporation, Arrowhead Pharmaceuticals, Inc., Benitec Biopharma, Inc., Alnylam Pharmaceuticals, Inc., Sanofi S.A. (Sanofi Genzyme), Biogen, Inc., Gradalis, Inc., Ionis Pharmaceuticals, Inc., Sarepta Therapeutics, Inc., and Silence Therapeutics Plc.
By Type
By Application
By End User
By Geography
The RNA based therapeutic market size is projected to reach USD 11.4 billion by 2027.
The rising popularity of personalized medicines are driving the market in coming years, however, obstacles in the delivery of mRNA limited the growth of the market.
Arbutus Biopharma Corporation, Arrowhead Pharmaceuticals, Inc., Benitec Biopharma, Inc., Alnylam Pharmaceuticals, Inc., Sanofi S.A. (Sanofi Genzyme), Biogen, Inc., Gradalis, Inc., Ionis Pharmaceuticals, Inc., Sarepta Therapeutics, Inc., and Silence Therapeutics Plc.
The Genetic disorders market is leading the Global RNA Based Therapeutic Market by Application 2020, and would continue to be a dominant market till 2027.
The Hospitals & Clinics market shows high growth rate of 17% during (2021 - 2027).
The North America segment dominated the Global RNA Based Therapeutic Market by Region 2020, and would continue to be a dominant market till 2027.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
