
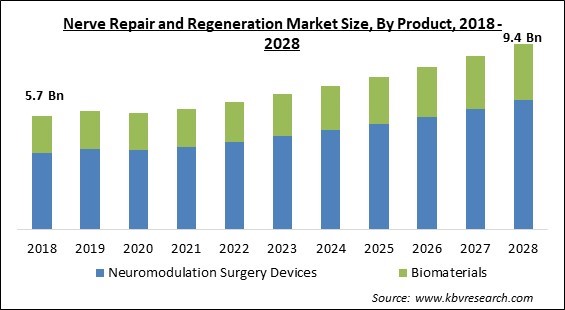
The Global Nerve Repair and Regeneration Market size is expected to reach $9.4 billion by 2028, rising at a market growth of 6.4% CAGR during the forecast period.
Neurostimulation, neuromodulators, and biomaterial devices are among the goods available for nerve repair and regeneration. These devices are used to treat traumatic or neurodegenerative conditions such Alzheimer's, Parkinson's, multiple sclerosis, amyotrophic lateral sclerosis, and multiple system atrophy. The spinal cord,brain, and peripheral nerves are all delicate and easily damaged, which can make it difficult for the brain to communicate with muscles and organs. These damages are repaired with nerve repair and regeneration products.
As in developing countries, the market opportunity for nerve repair and regeneration is growing, because healthcare spending continues to climb. The overall increase in awareness of the usage of nerve repair and regeneration devices to treat ailments including Parkinson's disease, Alzheimer's disease, and other neurological disorders is predicted to boost market growth. Furthermore, the nerve repair and regeneration market is expected to grow as individuals become more aware of therapeutic treatments such as immune response and neuroregeneration, spinal cord repair, peripheral nerve regeneration and repair, and nerve cell regrowth.
Remyelination post grade I lesions and collateral axon sprouting and proximal-to-distal nerve regeneration following grade II to grade V lesions are the two methods of nerve regeneration. Healing of function happens as the Schwann split happens and initiates remyelination in focal demyelinating lesions. Conduction, and hence strength, is restored in a matter of weeks or months, although the new myelin sheath is frequently thinner and has many internodes for each original internode.
The emergence of COVID-19 had a detrimental influence on the nerve repair and regeneration sector, as hospital and healthcare services were drastically decreased as a result of global social distancing measures. Furthermore, the COVID-19 pandemic had an economic impact and had a significant influence on normal hospital care for non-COVID-19 patients in hospitals all over the world. The need for nerve repair or regeneration procedures has decreased while numerous hospitals and clinics remain closed due to the lockdown.
With the large population bases, rising incidences of neurological illnesses, and growing healthcare infrastructure and expenditure, emerging economies like India, China, and Brazil offer lucrative new opportunities to the companies operating in this market. As various stem cell treatment studies under way for nerve repair and regeneration, the developing technologies of stem cell therapy also provide huge prospects for market expansion. In the last several years, research in the field of neurology has expanded and continues to increase.
Nerve repair and regeneration products are in high demand due to the rising frequency of neurological illnesses and the increased number of occurrences of nerve injury. The geriatric population is growing all across the world. The market's growth is fueled by the large geriatric population and the widespread frequency and incidence of nerve illnesses among them. Market players and researchers are concentrating their efforts on developing neurostimulation and neuromodulation technologies like next-generation neurostimulation devices, company collaborations to launch advanced products, and the introduction of new technologies, are propelling the market forward. The government's funding in neurological illness research is helping healthcare organizations to boost their research work and improve the efficacy of the nerve repair and regeneration products and procedures.
The market is confronted with a number of obstacles that are slowing its rate of expansion. There is a shortage of qualified experts who are required for neurological interventions due to a wide disparity between demand and supply of neurologists around the world. Tissue morbidity affects donor nerves frequently, which is a big problem. Neural gaps more than 3 cm are difficult to fix with nerve conduits. Neuromodulation and neurostimulation devices, for example, fall under Class II or Class III medical devices, depending on the application, and require premarket notification via the 510(k) process for clearance and marketing in the US market.
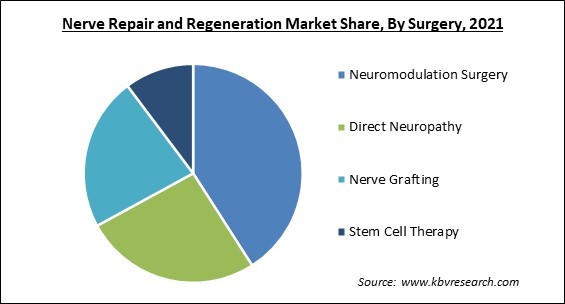
Based on Surgery, the market is segmented into Neuromodulation Surgery, Direct Neuropathy, Nerve Grafting, and Stem Cell Therapy. Stem Cell Therapy segment recorded a substantial revenue share in nerve repair and regeneration market in 2021. The market is expected to increase as a result of various government efforts and approvals to perform clinical studies of biomaterials. In the United States, there are roughly 570 clinics that provide stem cell therapies, and this number is likely to grow, boosting segment growth.
Based on Product, the market is segmented into Neuromodulation Surgery Devices and Biomaterials. The neuromodulation surgery devices segment acquired the largest revenue share in nerve repair and regeneration market in 2021. It is because of increased consumer awareness of nerve repair and new technology breakthroughs in the healthcare industry. In addition, the segment's expansion was fueled by a substantial number of commonly available spinal cord products and a diverse range of applications.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 6.1 Billion |
| Market size forecast in 2028 | USD 9.4 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 6.4% from 2022 to 2028 |
| Number of Pages | 167 |
| Number of Tables | 265 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Surgery, Product, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. North America emerged as a leading region in the nerve repair and regeneration market in 2021 with the largest revenue share. It is due to an increase in the occurrence of neurodegenerative and neurodevelopmental illnesses, stroke, and traumatic brain injuries, as well as an increase in patient awareness of nerve repair and regeneration techniques.
Free Valuable Insights: Global Nerve Repair and Regeneration Market size to reach USD 9.4 Billion by 2028
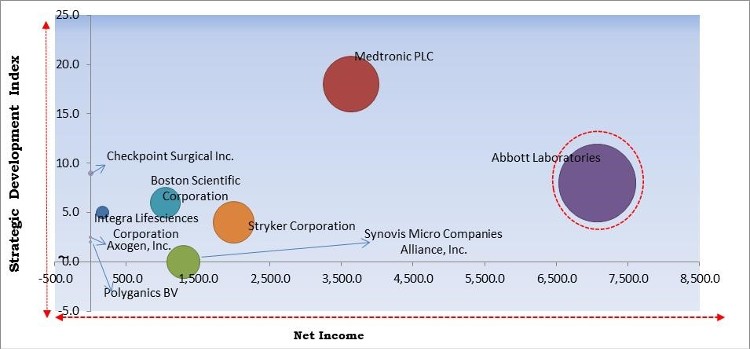
The major strategies followed by the market participants are Acquisitions. Based on the Analysis presented in the Cardinal matrix; Abbott Laboratories are the forerunners in the Nerve Repair and Regeneration Market. Companies such as Medtronic PLC, Boston Scientific Corporation and Stryker Corporation are some of the key innovators in the Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Abbott Laboratories, Boston Scientific Corporation, Integra Lifesciences Corporation, Medtronic PLC, Axogen, Inc., Stryker Corporation, Checkpoint Surgical, Inc., Polyganics BV, Synovis Micro Companies Alliance, Inc. (Baxter International, Inc.), and OrthoMed, Inc.
By Surgery
By Product
By Geography
The global nerve repair and regeneration market size is expected to reach $9.4 billion by 2028.
Rise in number of researches around the world are driving the market in coming years, however, lack of skilled healthcare professionals growth of the market.
Abbott Laboratories, Boston Scientific Corporation, Integra Lifesciences Corporation, Medtronic PLC, Axogen, Inc., Stryker Corporation, Checkpoint Surgical, Inc., Polyganics BV, Synovis Micro Companies Alliance, Inc. (Baxter International, Inc.), and OrthoMed, Inc.
The Neuromodulation Surgery segment acquired maximum revenue share in the Global Nerve Repair and Regeneration Market by Surgery in 2021, thereby, achieving a market value of $4.69 billion by 2028.
The North America market dominated the Global Nerve Repair and Regeneration Market by Region in 2021, and would continue to be a dominant market till 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
