
The Global Mycoplasma Testing Market size is expected to reach $1.9 billion by 2030, rising at a market growth of 11.5% CAGR during the forecast period.
The increasing awareness of the impact of mycoplasma contamination on cell culture research outcomes drives the growth of this testing in academic settings. Academic research institutes heavily rely on cell cultures for various studies, from basic cell biology to disease modeling and drug discovery. Consequently, the academic research institutes segment would acquire nearly 15 % of the total market share by 2030. Researchers prioritize implementing this testing to maintain the reliability of their experiments. Developing tissue engineering and three-dimensional (3D) cell culture models in academic research requires stringent quality control measures, including this testing. As researchers explore advanced cell culture techniques, the demand for these testing solutions that accommodate diverse models and experimental setups increases.

Advances in these testing methods, including rapid and high-throughput technology development, contribute to the market's growth. Technological innovations make testing more efficient, sensitive, and cost-effective, driving adoption across various industries. Mass spectrometry-based approaches, like matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), are gaining traction in mycoplasma identification. These techniques provide high-resolution and accurate identification of microbial species, including mycoplasma. Hence, the market is expanding significantly due to the technological advancements in testing methods. Additionally, the growth of the biopharmaceutical and biotechnology industries, characterized by the increasing production of therapeutic proteins, monoclonal antibodies, and other biologics, fuels the demand for this testing. The increasing production of biosimilars and biological products like approved biopharmaceuticals contributes to the demand for this testing. Thus, because of the rising biopharmaceutical and biotechnology industries, the market is anticipated to increase significantly.
Moreover, the COVID-19 pandemic intensified the global focus on vaccine development and biopharmaceutical production. With accelerated efforts to produce vaccines and therapeutic biologics, the demand for these testing solutions increased to ensure the quality and safety of these products. The urgency to develop and produce vaccines and therapeutics during the pandemic led to increased regulatory scrutiny. Regulatory agencies emphasized the importance of stringent quality control measures, including this testing, to ensure the safety and efficacy of emerging treatments. Thus, the COVID-19 pandemic had a moderate effect on the market.
However, Variations in results may result from the absence of standardized testing procedures among laboratories and regions. Achieving a universal standard for this testing is challenging due to the diversity of testing techniques and technologies used in different settings. Standardization may lead to complacency in continuously improving these testing methodologies. The assumption that a standardized method is universally applicable may discourage efforts to enhance testing technologies and protocols further. Hence, standardization of testing methods is a significant challenge that hampers the growth of market.
 Drivers
Drivers  Restraints
Restraints Based on technology, the market is classified into PCR, ELISA, microbial culture techniques, and enzymatic methods. The microbial culture techniques segment acquired a substantial revenue share in the market in 2022. Culturing mycoplasma isolates allows species identification, allowing laboratories to determine the specific types of mycoplasma present. This information can be valuable for understanding the nature of contamination and tailoring appropriate responses. Microbial culture techniques enable the testing of viable mycoplasma organisms, providing information on their ability to replicate and cause harm to cell cultures or biopharmaceutical products. This aspect is crucial for understanding the potential impact of contamination on production processes.
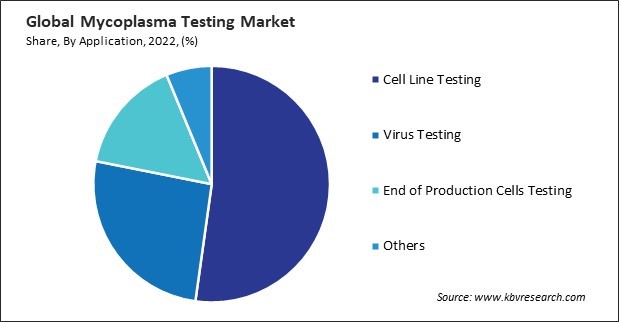
On the basis of application, the market is divided into cell line testing, virus testing, end of production cells testing, and others. The virus testing segment garnered a significant revenue share in the market in 2022. Virus testing, alongside this testing, helps prevent cross-contamination in shared laboratory spaces and manufacturing facilities. The risk of cross-contamination between different cell lines or production batches is a concern that both types of testing address to maintain the integrity of biological products. Thorough virus and this testing helps to build public trust in the safety and efficacy of pharmaceutical and biopharmaceutical products. Ensuring that these products are free from harmful contaminants is paramount for the safety of patients and consumers.
By end user, the market is segmented into academic research institutes, cell banks, contract research organizations, pharmaceutical & biotechnology companies, and others. The contract research organizations segment recorded a remarkable revenue share in the market in 2022. Contract research organizations (CROs) conduct preclinical and clinical studies for pharmaceutical and biotechnology companies. In these studies, this testing is essential to ensure the integrity of cell cultures and prevent contamination that can affect the reliability of study outcomes. CROs are early adopters of technological advancements in these testing methods. Their commitment to staying at the forefront of testing technologies drives the adoption of innovative and efficient mycoplasma detection methods.
By product & services, the market is categorized into instruments, kits & reagents, and services. The instruments segment covered a considerable revenue share in the market in 2022. Polymerase chain reaction (PCR) machines are widely used in this testing for amplifying and detecting specific DNA sequences. PCR-based assays are highly sensitive and specific, allowing for the rapid and accurate identification of mycoplasma DNA in samples. Microscopes, including fluorescent microscopy systems, are used to observe mycoplasma contamination in cell cultures directly. Fluorescent dyes are applied to label mycoplasma cells, making them visible under a microscope. This method allows for quick and direct visualization of contamination.
Free Valuable Insights: Global Mycoplasma Testing Market size to reach USD 1.9 Billion by 2030
Region-wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. In 2022, the North America region led the market by generating the highest revenue share. The biopharmaceutical industry in North America strongly emphasizes maintaining high manufacturing standards. Academic and research institutions in North America are actively involved in cell culture research, ranging from basic cell biology to advanced applications in biotechnology. The region is an early adopter of advanced testing technologies, including rapid and high-throughput methods for mycoplasma detection.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 790.4 Million |
| Market size forecast in 2030 | USD 1.9 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 11.5% from 2023 to 2030 |
| Number of Pages | 290 |
| Number of Tables | 480 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Porter’s 5 Forces Analysis, Company Profiling, Companies Strategic Developments, SWOT Analysis, Winning Imperatives |
| Segments covered | Product & Service, Technology, Application, End User, Region |
| Country scope |
|
| Companies Included | Charles River Laboratories International, Inc., Thermo Fisher Scientific, Inc., Eurofins Scientific SE, Lonza Group Ltd., Bio-Rad Laboratories, Inc., InvivoGen SAS, Asahi Kasei Corporation, F. Hoffmann-La Roche Ltd., Norgen Biotek Corp. and PromoCell GmbH |
By Product & Service
By Technology
By Application
By End User
By Geography
The Market size is projected to reach USD $1.9 billion by 2030.
Technological advancements in testing methods are driving the Market in coming years, however, Standardization of testing methods restraints the growth of the Market.
Charles River Laboratories International, Inc., Thermo Fisher Scientific, Inc., Eurofins Scientific SE, Lonza Group Ltd., Bio-Rad Laboratories, Inc., InvivoGen SAS, Asahi Kasei Corporation, F. Hoffmann-La Roche Ltd., Norgen Biotek Corp. and PromoCell GmbH
The expected CAGR of this Market is 11.5% from 2023 to 2030.
The Kits & Reagents segment generated the highest revenue in the Market by Product & Service in 2022; thereby, achieving a market value of $933.4 million by 2030.
The North America region dominated the Market by Region in 2022, and would continue to be a dominant market till 2030; thereby, achieving a market value of $756.2 million by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
