
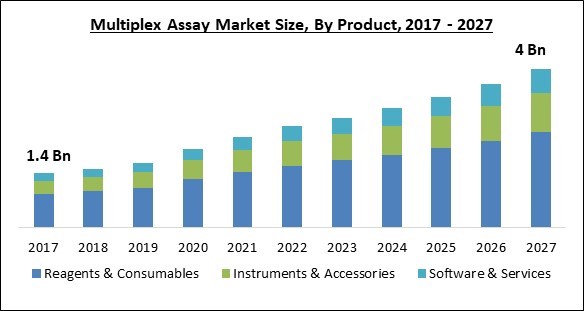
The Global Multiplex Assay Market size is expected to reach $4 billion by 2027, rising at a market growth of 9.8% CAGR during the forecast period.
A multiplex assay is a process that detects and quantifies many analytes such as biomolecules, proteins, cytokines, growth factors, and chemokines at the same time. In comparison to traditional approaches like ELISA, this assay is used to amplify many objectives in a polymerase chain reaction (PCR) and capture more details from minute amounts of proteins or other analytes in less time. Pathogen identification, RNA detection, gene detection analysis, linkage analysis, mutation analysis, and forensic studies all use multiplex assays.
Running numerous tests means there may be little differences within every run, which could affect the results. Running a single experiment prevents this fluctuation from having a large impact, thus any differences in the protein found to be more likely to indicate genuine relative abundance within the sample. One such disease is sepsis, which is defined by a shift in the levels of pro-inflammatory cytokines.
Certain chemicals, some of which have been explored as possible medicinal medications, can also alter cytokine levels. As a result, having a system that can assess the levels of many cytokines at the same time is advantageous. Each bead had captured antibodies for the above cytokines and was differentiated by the ratio of dye added in a bead-based approach.
In addition, high-throughput multiplex detection techniques are used to evaluate a large number of analytes such as nucleic acid assays, immunoassays, enzyme assays, or receptor ligands, in a single biological sample in a fast, sensitive, and specific manner. These methods allow the analysis of a huge number of samples in one go.
The COVID-19 pandemic has wreaked havoc on healthcare workflows all around the world. Numerous industries, including plenty of sub-domains of health care, have been compelled to temporarily shut down their production facilities because of the disease. Since multiplex assays are used to diagnose coronaviruses, there has been a substantial demand for multiplex SARS-CoV-2 assays, such as multiplex point-of-care assays, which have helped the multiplex assay market grow during the pandemic. The link of COVID-19 with multiple hazardous disorders increased the utilization of multiplex assays across the market.
In addition, to address the growing demand of the high volume of testing, several businesses got FDA emergency use authorization for their COVID-19 multiplex assays. However, the companies had to deal with several operational issues that hindered their commercial operations throughout the lockdown.
Companion diagnostics method is rapidly emerging and being utilized in therapeutics. A companion diagnostic is a diagnostic test that is used in conjunction with a therapeutic drug to determine whether the specific personalized medicine is appropriate for a particular person. Companion diagnostics assays can be conducted on a tissue biopsy or a blood sample utilizing various genomic or protein-based technologies such as next Generation Sequencing, qPCR, and Immunohistochemistry. In the case of cancer, a companion diagnostic test can determine whether a patient's tumor has a specific genetic aberration, such as a mutation, or changed protein expression, that predicts better therapeutic medication efficacy.
There is an increased usage of multiplex assays in companion diagnostics, as well as their advantages over singleplex & classical assays is the result of the growing prevalence of chronic diseases. The advantage of multiplex assays over singleplex tests, which includes lower reaction tie, chemicals, and analyte concentration, allows for faster assay results and the handling of unusual samples (low concentration sample). The high frequency of infectious and chronic diseases creates greater market potential through increasing demand for multiplex assays.
Multiplex assays comprise the potential to bring a new evolution across the healthcare industry and can play a major role in the developments and enhancements in medications that are being utilized in the treatment of numerous diseases and disorders such as cancer and neurological diseases. However, the equipment that is employed in the utilization of multiplex assays is very costly. Due to the higher cost of the equipment, the researchers or healthcare institutes that comprise lesser financial power would find it difficult to purchase and employ these products.
Based on the Product, the Multiplex Assay Market is segregated into Reagents & Consumables, Instruments & Accessories, and Software & Services. In 2020, the Instruments & Accessories segment procured the maximum revenue share of the multiplex assays market. The industry standard multiplexing instruments and accessories are capable of performing multiple distinct tests in a single reaction volume. For instance, the flow cytometry-based equipment is capable of producing quick and cost-effective findings. These factors are majorly driving the growth of this segment.
Based on the Type, the Multiplex Assay Market is segmented into Protein Based Multiplex Assays, Nucleic Acid-Based Multiplex Assay, and Other multiplex Assays. In 2020, the Nucleic Acid-Based Multiplex Assay segment acquired a substantial revenue share of the multiplex assays market. It is owing to the significant features of the nucleic acid-based multiplex assays. Nucleic acid-based approaches are generally precise, and they may be used for a wide range of microorganisms. Because each test is often exclusive to an individual organism, the physician must be aware of the diagnostic options and make appropriate test requests.
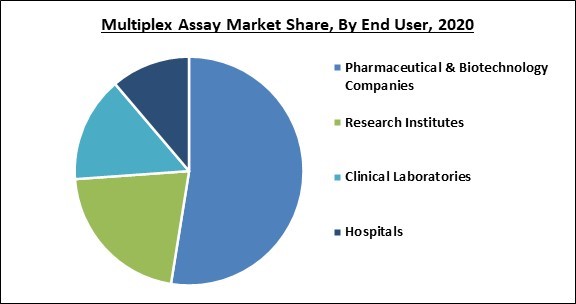
Based on the End-user, the Multiplex Assay Market is categorized into Hospitals, Clinical Laboratories, Research Institutes, and Pharmaceutical & Biotechnology Companies. In 2020, the research institutes garnered a significant revenue share in the multiplex assays market. Multiplex assay methods are widely utilized in research and development operations. The rising utilization of the multiplex assays across research institutes is owing to the fact that multiplex assays provide convenience and faster results when running experiments.
Based on the Application, the Multiplex Assay Market is divided into Clinical Diagnostics, Research & Development, and Companion Diagnostics. In 2020, the companion diagnostics segment procured the second-largest revenue share of the multiplex assays market. The growth of this segment is attributed to the fact that the test facilitates a practitioner to determine if the benefits of a drug combination can cause any potentially critical side effects to patients or not. Moreover, companion diagnostics tests allow healthcare practitioners to determine the efficiency of the personalized combination.
| Report Attribute | Details |
|---|---|
| Market size value in 2020 | USD 2 Billion |
| Market size forecast in 2027 | USD 4 Billion |
| Base Year | 2020 |
| Historical Period | 2017 to 2019 |
| Forecast Period | 2021 to 2027 |
| Revenue Growth Rate | CAGR of 9.8% from 2021 to 2027 |
| Number of Pages | 269 |
| Number of Tables | 454 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Product, Type, Application, End User, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on the Region, the Multiplex Assay Market is analyzed across North America, Europe, APAC, and LAMEA. In 2020, North America emerged as the leading region in the multiplex assays market with the largest revenue share. The increased growth of the segment is attributed to the rise in the number of research institutes. Due to the increasing interest of North American researchers toward discovering more potentials of these immunoassays for making treatment of various diseases and disorders more efficient.
Free Valuable Insights: Global Multiplex Assay Market size to reach USD 4 Billion by 2027
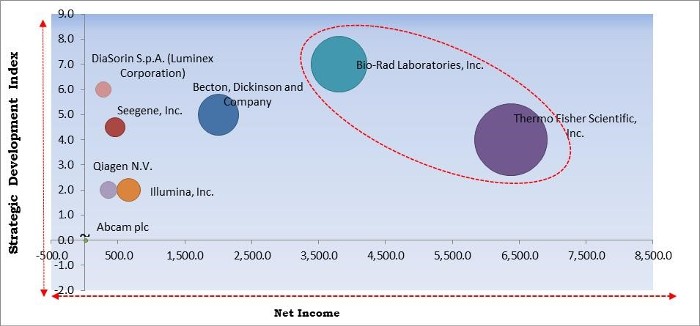
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; Thermo Fisher Scientific, Inc. and Bio-Rad Laboratories, Inc. are the forerunners in the Multiplex Assay Market. Companies such as Becton, Dickinson and Company, Seegene, Inc. and DiaSorin S.p.A. (Luminex Corporation) are some of the key innovators in the Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., Becton, Dickinson and Company, Seegene, Inc., Randox Laboratories Limited, Illumina, Inc., Qiagen N.V., DiaSorin S.p.A. (Luminex Corporation), Abcam plc, and Meso Scale Diagnostics LLC.
By Product
By Type
By End User
By Application
By Geography
The global multiplex assay market size is expected to reach $4 billion by 2027.
Increasing popularity of companion diagnostics are driving the market in coming years, however, rising equipment costs and stringent regulations and standards limited patents limited the growth of the market.
Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc., Becton, Dickinson and Company, Seegene, Inc., Randox Laboratories Limited, Illumina, Inc., Qiagen N.V., DiaSorin S.p.A. (Luminex Corporation), Abcam plc, and Meso Scale Diagnostics LLC.
The Reagents & Consumables market acquired maximum revenue share in the Global Multiplex Assay Market by Product 2020, achieving a market value of $2.4 billion by 2027.
The Protein Based Multiplex Assays market shows high growth rate of 9.4% during the forecast period.
The North America market is the fastest growing region in the Global Multiplex Assay Market by Region 2020, and would continue to be a dominant market till 2027.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
