
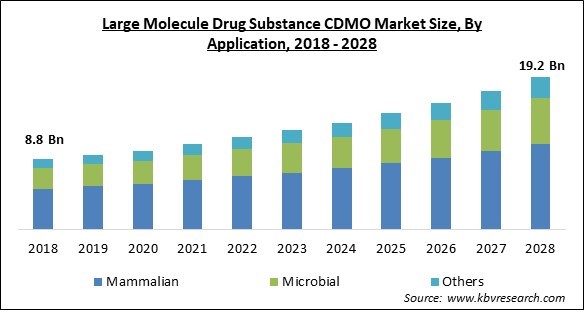
The Global Large Molecule Drug Substance CDMO Market size is expected to reach $19.2 billion by 2028, rising at a market growth of 8.7% CAGR during the forecast period.
Contract development and manufacturing organization (CDMO) that provide services for processing large molecule or biologics drug substances are referred to as large molecule drug substance CDMO. These are companies that assist customers in drug discovery via manufacturing abilities by providing services and, thus, also help the pharmaceutical industry.
Large molecules are proteins that tend to have a therapeutic effect. These are highly complex, and most biologics are composed of approximately 1300 or more amino acids. These are prepared so that they resemble human proteins. The rise in molecule drug approvals, growing infectious disease prevalence, and increasing favorability for innovative therapeutics demand more CDMO setups for the rapid development and manufacturing of drugs.
Therefore, CDMOs' research, knowledge, and manufacturing abilities are highly necessary to proceed with drug development. As such, many CDMOs engage in integrating third-party ventures, which are also propelled by biotech and pharma companies' assistance through financial and technological assets. Most customers also expect CDMOs to offer their expertise even after manufacturing, like in commercial launches.
This is mainly because CDMO’s customer base has expanded beyond the big pharmaceutical companies to smaller biotech firms. Additionally, many smaller biotech companies focus extensively on evolving their drug pipelines without any manufacturing experience. Therefore, taking help from CDMOs in development and manufacturing operations also enables the early incorporation of smaller biotech firms’ operations.
Some governments imposed regulations that resulted in the procurement of sufficient volumes of therapeutics and vaccines that were produced locally. However, production capacities were still not at their maximum because of supply chain and lockdown constraints. Disrupted supply chains interfered with the procurement of goods needed in the production processes, like the single-use plastic bags, which CDMOs use in the reactors. In addition, the lockdown constraints introduced a reduction in the workforce. These constraints resulted in a significant increase in the lead times, which increased up to four times. The delayed production and increased demand further helped in the growth of the CDMOs. Therefore, the pandemic positively impacted the large molecule drug substance CDMO market.
Since the pandemic, numerous businesses have been making significant investments in the creation of biologics and biosimilar compounds. Presently, biologics such as peptides, proteins, and monoclonal antibodies in the discovery stage are making up almost half of the candidates intended to be used as therapeutics. Biopharmaceutical businesses are increasingly putting their resources into research and development, and as a result, many novel biologics are either being developed or are in the pipeline. Hence, all of these factors aid in the growth of the large molecule drug substance CDMO market.
According to a paper published in the Journal of the American College of Cardiology that examined the total severity of cardiovascular disease (CVD) burden, the amount of people who die from (CVD) is steadily increasing. This number also includes one-third of all deaths globally in 2019. The statistics highlight the urgent need for nations to set up affordable public health initiatives that are affordable and focused on lowering heart disease risk through modifiable behaviors.
The characterization of large molecules is extremely challenging. Biopharmaceutical companies are needed to characterize in numerous ways, including stability, purity, and function. Large molecules routinely demand a combination of many low and high-resolution techniques to validate the particle's structure efficiently. Additionally, numerous post-translational modifications (PTMs) are produced by large molecules during the recombinant synthesis method. Hence, the rise in R&D and investments in product development are boosting the growth and advancement of the large molecule drug substance CDMO market.
Based on application, the large molecule drug substance CDMO market is categorized into mammalian, microbial, and others. The microbial segment procured a considerable growth rate in the large molecule drug substance CDMO market in 2021. Microbial systems are mostly utilized in the production of recombinant proteins to be used in therapeutic processes. As most of the drugs available in the market are produced through recombinant technologies, they propel the growth of microbial systems.
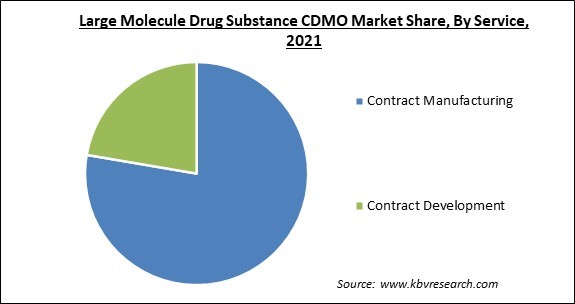
On the basis of service, the large molecule drug substance CDMO market is divided into contract manufacturing and contract development. The contract manufacturing segment acquired the largest revenue share in the large molecule drug substance CDMO market in 2021. Contract manufacturing performs various functions like target screening and identification, functional informatics and target validation, preclinical development, and candidate optimization and lead identification.
Based on end-user, the large molecule drug substance CDMO market is segmented into biotech companies, CRO, and others. The CRO segment witnessed a considerable growth rate in the large molecule drug substance CDMO market in 2021. The clinical research organization needs the CDMOs assistance with the laboratory, clinical, preclinical, and discovery services. In addition, the rising demand for treatments of various diseases and disorders has also increased the volume of research to be conducted.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 10.8 Billion |
| Market size forecast in 2028 | USD 19.2 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 8.7% from 2022 to 2028 |
| Number of Pages | 278 |
| Number of Tables | 501 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Application, Service, End-user, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
On the basis of region, the large molecule drug substance CDMO market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The Asia Pacific segment garnered the maximum revenue share in the large molecule drug substance CDMO market in 2021. A skilled workforce, compliable regulations, and lower overall costs are the main factors that propel the outsourcing of large molecule drug substances to the CDMOs of this region. The presence of a considerable consumer base present in this region has increased the need for improved medicines and treatment techniques.
Free Valuable Insights: Global Large Molecule Drug Substance CDMO Market size to reach USD 19.2 Billion by 2028
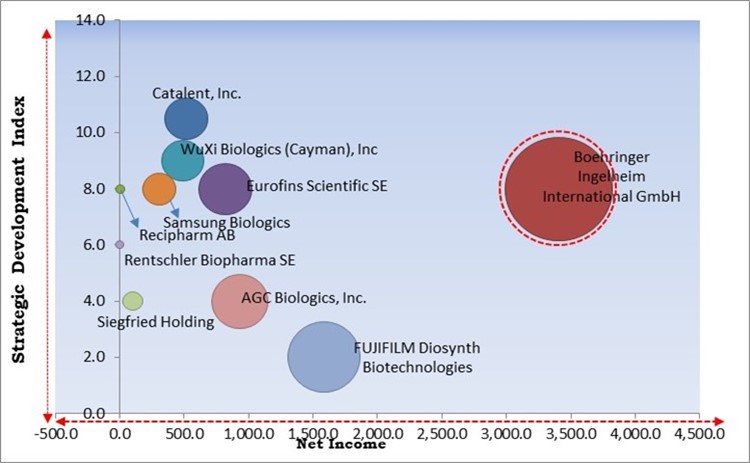
The major strategies followed by the market participants are Partnerships. Based on the Analysis presented in the Cardinal matrix; Boehringer Ingelheim International GmbH is the forerunner in the Large Molecule Drug Substance CDMO Market. Companies such as FUJIFILM Diosynth Biotechnologies Texas LLC, WuXi Biologics (Cayman), Inc., Eurofins Scientific SE are some of the key innovators in Large Molecule Drug Substance CDMO Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Eurofins Scientific SE, WuXi Biologics (Cayman), Inc, Catalent, Inc., Boehringer Ingelheim International GmbH, FUJIFILM Diosynth Biotechnologies Texas LLC (Fujifilm Corporation), Samsung Biologics Co., Ltd. (Samsung Group), Rentschler Biopharma SE, AGC Biologics, Inc. (AGC, Inc.), Recipharm AB (EQT AB), and Siegfried Holding AG.
By Application
By Service
By End-user
By Geography
The global Large Molecule Drug Substance CDMO Market size is expected to reach $19.2 billion by 2028.
Increasing Research And Development Of Biologics And Biosimilars are driving the market in coming years, however, Complex Analytical Characterization Of Biologics And Strict Parameters And Regulations restraints the growth of the market.
Eurofins Scientific SE, WuXi Biologics (Cayman), Inc, Catalent, Inc., Boehringer Ingelheim International GmbH, FUJIFILM Diosynth Biotechnologies Texas LLC (Fujifilm Corporation), Samsung Biologics Co., Ltd. (Samsung Group), Rentschler Biopharma SE, AGC Biologics, Inc. (AGC, Inc.), Recipharm AB (EQT AB), and Siegfried Holding AG.
The Mammalian segment acquired maximum revenue share in the Global Large Molecule Drug Substance CDMO Market by Application in 2021 thereby, achieving a market value of $10.8 billion by 2028.
The Biotech Companies segment is leading the Global Large Molecule Drug Substance CDMO Market by End-user in 2021 thereby, achieving a market value of $8.6 billion by 2028.
The Asia Pacific market dominated the Global Large Molecule Drug Substance CDMO Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $7 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
