
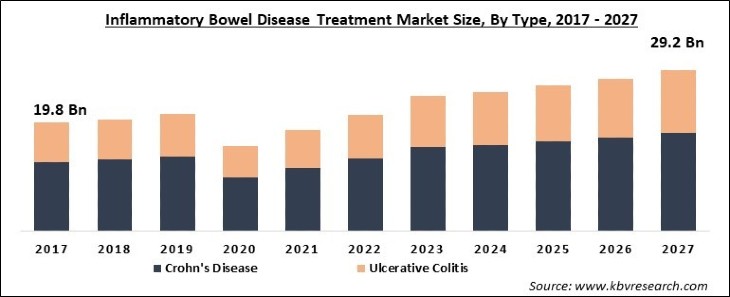
The Global Inflammatory Bowel Disease Treatment Market size is expected to reach $29.2 billion by 2027, rising at a market growth of 8.1% CAGR during the forecast period. Inflammatory bowel disease refers to a group of intestinal disorders that leads to chronic inflammations like swelling and pain in the intestines. Few of the known inflammatory bowel disease are Ulcerative colitis and Crohn’s disease. The effect of both types of inflammatory bowel disease is witnessed on the digestive system. There are various treatments available that only help to slow down the progress of the disease. The kind of treatment required for inflammatory bowel disease largely depends upon symptoms and the type of IBD. Moreover, the prescribed medicines assist patients in controlling inflammation.
The key factor contributing to the growth of the market is the growing prevalence of ulcerative disease and Crohn’s disease around the world. Additionally, the presence of strong pipeline drugs like risankizumab, ustekinumab, tofacitinib, & upadacitinib, and the growing adoption of biologics for the inflammatory bowel disease treatment are projected to accelerate the growth of the market in the upcoming period. However, the outbreak of the COVID-19 has an adverse effect on the growth of the market as a rapid decrease was observed in the diagnosis of inflammatory bowel disease, owing to the imposition of lockdown in several countries around the world.
The outbreak of the COVID-19 pandemic had a negative impact across the world. During the Quarter 2 of 2020, there was a rapid increase witnessed in the number of COVID-19 patients due to which, WHO declared it as a Public Health Emergency. Governments across the world formed rules to reduce the impact of the COVID-19. Lockdown was imposed that led to the shutdown of businesses, which had a negative impact on the economies of nations. Traveling was banned that disrupted the supply chains. People were forced to stay at their homes and maintain social distance.
As the prevalence of inflammatory bowel disease is increasing rapidly, governments across several nations are focusing on finding efficient and less painful treatments for IBD patients. Thus, governments are highly investing in research and development activities to develop new products that will provide relief to patients suffering from inflammatory bowel disease. Moreover, the government is also providing reimbursement policies to patients due to which, a large number of patients are adopting IBD treatment.
As the world is evolving, most of the nations are shifting towards urbanization. People residing in different nations are largely following modern culture and trending activities. People are also inclining towards a luxurious life, which has given rise to an unhealthy lifestyle. In addition, the advent of advanced technologies has made people lazy and inactive. Additionally, people are adopting unhealthy activities such as smoking and consuming alcohol. The unhealthy lifestyle has resulted in giving rise to various health-related problems like inflammatory bowel disease.
The guidelines for the British Society of Gastroenterology were developed on the management of inflammatory bowel disease in youngsters. The major UK authorities of Ulcerative colitis and Crohn’s disease healthcare were involved in the development of guidelines. These guidelines were imposed at the time of rapid change in several factors of inflammatory bowel disease. The imposition of stringent guidelines has created barriers to the growth of the inflammatory bowel disease treatment market.
Based on Type, the market is segmented into Crohn's Disease and Ulcerative Colitis. In 2020, the Crohn's Disease segment acquired the largest revenue share of the inflammatory bowel disease treatment market and would continue the progress during the forecast period. The major factors accelerating the growth of the segment are the growing rate of biologics prescription and the high prevalence rate.
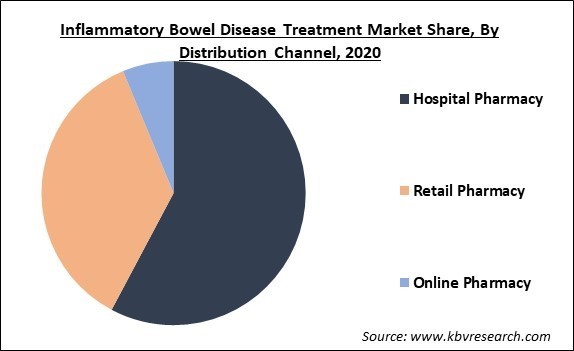
Based on Distribution Channel, the market is segmented into Hospital Pharmacy, Retail Pharmacy and Online Pharmacy. In 2020, the hospital pharmacy segment collected the maximum revenue share of the inflammatory bowel disease treatment market and is expected to display similar kind of trend even during the forecast years. The factors contributing to the growth of the segment are rapidly growing hospitalization of inflammatory bowel disease patients and higher cost of biologics.
Based on Route of Administration, the market is segmented into Injectable and Oral. The oral segment is anticipated to display a prominent CAGR during the forecasting years. Factors such as the presence of robust oral pipeline products to treat patients suffering from inflammatory bowel disease and the approval of JAK inhibitors for inflammatory bowel disease treatment are responsible for the growth of this segment. Moreover, the forthcoming launch of S1P modulators and JAK inhibitors will propel the growth of the market over the forecast period.
Based on Drug Class, the market is segmented into TNF inhibitors, Anti-integrin, IL inhibitors, Corticosteroids, JAK inhibitors, Aminosalicylates and Others. The drug segment like aminosalicylates, corticosteroids, and anti-integrin are displaying a prominent share in the inflammatory bowel disease treatment market. Aminosalicylates refer to the first-line therapy for inflammatory bowel disease treatment. JAK inhibitors segment would showcase the fastest growth rate during the forecasting years.
| Report Attribute | Details |
|---|---|
| Market size value in 2020 | USD 15.5 Billion |
| Market size forecast in 2027 | USD 29.2 Billion |
| Base Year | 2020 |
| Historical Period | 2017 to 2019 |
| Forecast Period | 2021 to 2027 |
| Revenue Growth Rate | CAGR of 8.1% from 2021 to 2027 |
| Number of Pages | 288 |
| Number of Tables | 464 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Type, Distribution Channel, Route of Administration, Drug Class, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. Asia-Pacific would exhibit the fastest CAGR of inflammatory bowel disease treatment during the forecasting years. The growth of the regional market would be driven by the increasing novel drug approval, the growing prevalence of ulcerative colitis and Crohn’s disease across the region, and increasing prescription of biosimilars. Based on a study by NCBI, in comparison to women, the chances of Crohn’s disease are higher in men.
Free Valuable Insights: Global Inflammatory Bowel Disease Treatment Market size to reach USD 29.2 Billion by 2027
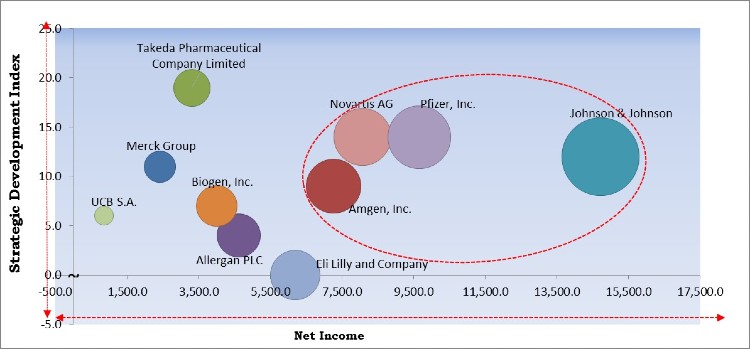
The major strategies followed by the market participants are Approvals. Based on the Analysis presented in the Cardinal matrix; Pfizer, Inc., Amgen, Inc., Novartis AG, and Johnson & Johnson are the forerunners in the Inflammatory Bowel Disease Treatment Market. Companies such as Takeda Pharmaceutical Company Limited, UCB S.A., Allergan PLC are some of the key innovators in the market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Allergan PLC (AbbVie), Johnson & Johnson, Merck Group, Amgen, Inc., Eli Lilly and Company, Biogen, Inc., Pfizer, Inc., Novartis AG, Takeda Pharmaceutical Company Limited, and UCB S.A.
By Type
By Distribution Channel
By Route of Administration
By Drug Class
By Geography
The inflammatory bowel disease treatment market size is projected to reach USD 29.2 billion by 2027.
Rapid urbanization led to an unhealthy lifestyle are driving the market in coming years, however, Strict regulatory policies for IBD drugs have limited the growth of the market.
Allergan PLC (AbbVie), Johnson & Johnson, Merck Group, Amgen, Inc., Eli Lilly and Company, Biogen, Inc., Pfizer, Inc., Novartis AG, Takeda Pharmaceutical Company Limited, and UCB S.A.
The expected CAGR of the inflammatory bowel disease treatment market is 8.1% from 2021 to 2027.
In 2020, the injectable segment garnered the highest market share of the inflammatory bowel disease treatment market. This is attributed to the high effectiveness of injectable drugs.
In 2020, North America emerged as the leading region in the inflammatory bowel disease treatment market by acquiring the largest revenue share and is anticipated to continue its dominance during the forecasting years.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
