
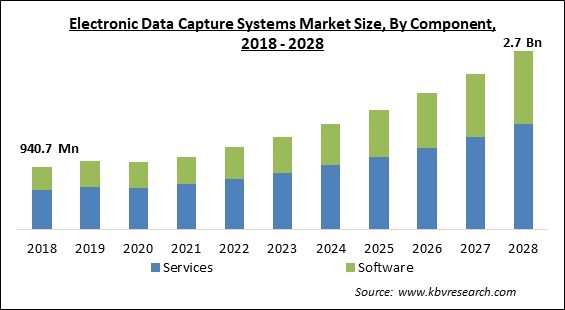
The Global Electronic Data Capture Systems Market size is expected to reach $2.7 billion by 2028, rising at a market growth of 13.8% CAGR during the forecast period.
An electronic data capture (EDC) system, or an electronic case report form (eCRF), is a highly essential software tool that is used in clinical trials. Both of these terms relate to the same thing. EDC solutions are used in order to gather, clean, and analyze the data that is created in clinical trials. Accurate data is essential for clinical research.
You will gain knowledge about the technical and practical elements of EDC systems, including the expenses associated with their purchase and use in clinical trials, by reading the following article. When it comes to running a clinical study, using an EDC system unquestionably offers a number of benefits that cannot be ignored.
To begin, implementing an EDC solution to gather data as opposed to collecting data via paper-based methods is much more efficient. The EDC system allows clinical sites to input data quickly and simply from any computer, making it instantly accessible to data reviewers. Clinical sites have access to the EDC system from any computer.
This eliminates the headache that comes with filling out and sending in paper forms. The process of data cleansing is made more efficient by EDC technologies. Data managers are able to readily check the data that was submitted into the system and send queries to sites in order to resolve any inconsistencies that may have been found. In addition, the integration of sophisticated methods to manage access restrictions and data traceability is another way in which an EDC platform ensures the authenticity, integrity, and safety of the data it stores.
As a result, it is projected that the COVID-19 pandemic will have a beneficial effect on the growth that is anticipated for the market for eClinical solutions. Additionally, it is anticipated that the COVID-19 outbreak will both hasten this pattern and result in long-term modifications to traditional trial management standards in the clinical trials market, which is moving toward digital work procedures. This is due to the fact that COVID-19 is a highly contagious virus. When the practicality of virtual and remote working arrangements for the conduct of trials is proved, it may be to the advantage of all providers of eClinical solutions to realize significant gains. As a consequence of this, the market for electronic data capture devices would profit from the widespread distribution of COVID-19.
One of the reasons that are driving the market for eClinical solutions is the growth in the sickness load that is prompting the creation of new medications. eClinical solutions improve the visibility of the data, which in turn makes the decision-making process go more quickly. The fragmentation of data and the difficulty in making decisions are both problems that may be solved by streamlining operational data management. Technology is designed to bring up new opportunities in clinical trials and to push the expansion of eClinical solutions by solving issues with data management via the automation of drive and by making the process of data driving easier through the use of eClinical solutions.
Streamlining processes, which leads to clinical tests that are completed more quickly and with more effectiveness, is one of the primary advantages of using electronic data-capturing systems in clinical trials. Because illnesses change on a daily basis, there is a growing need for the conduct of rapid clinical trials in order to find treatments and medications that may combat these diseases. The legal process can take many years. As a result, there is an increased need for exhaustive testing, clinical studies, and comprehensive assessments to determine whether or not medications and vaccines are safe and effective.
Because of advancements in technology and artificial intelligence, clinical trial sponsors and clinical research organizations (CROs) are modifying the ways in which they conduct their tests. They make it possible for researchers to collect an enormous amount of data and assess it in a more complete and expedient manner. Big data and artificial intelligence both offer a lot of potential benefits, but there are also some potential drawbacks. One sort of digital danger consists of hackers with the intention of stealing patient information and corrupting data. As a result, there needs to be a considerable amount of emphasis placed on cybersecurity.
Based on delivery mode, the electronic data capture systems market is classified into on-premise and, web & cloud-based. In 2021, the on-premise segment garnered the significant revenue share in the market. On-premises solutions provide increased levels of data protection and management, in addition to improved operational efficiencies. However, they entail substantial costs of implementation, which could be a factor in restraining the development of the category. It is anticipated that an expanding number of businesses will drive the expansion of the market.
Based on Component, the electronic Data Capture Systems Market is divided into Services and Software. The service segment led the electronic data capture system market in 2021, with the biggest revenue share. Through the automation of data input and the reduction of mistakes that it causes, EDC may help you save both time and money. In addition to this, it may assist you in enhancing the quality of your data by offering real-time validation and audit trails. In addition to this, EDC can assist you in meeting regulatory obligations by providing a safe and tamper-proof method for the collection and storage of data.
Based on development phase, the electronic data capture systems market is segregated into phase I, phase II, phase III, and phase IV. In the electronic data capture systems market in 2021, the Phase III segment generated the highest revenue share. This comes as a result of the strong demand for EDC software, which is intended to reduce overall costs and enhance the effectiveness of operations. On the other hand, Phase I is anticipated to expand at a profitable pace due to the fact that these systems assist in the analysis of clinical data, future outcomes, and the elimination of drug candidates that have a low likelihood of succeeding in clinical trials.
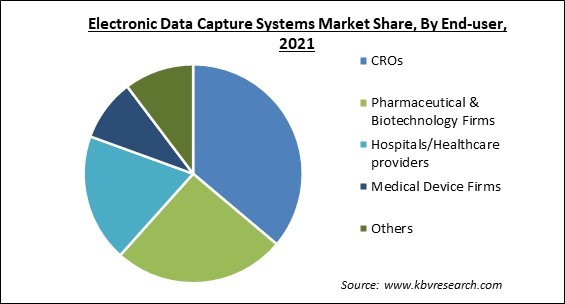
On the basis of End-user, the electronic data capture systems market is segmented into CROs, Pharmaceutical & Biotechnology Firms, Hospitals/Healthcare providers, Medical Device Firms, and Others. In 2021, the Hospitals/ Healthcare providers segment showcased the significant revenue share. This is a result of these end-users increased usage of EDC systems and services. It is critical to have accurate patient data in order to provide appropriate treatment and monitoring for patients. All of your data entry requirements may be met in a timely way with the assistance of a specialized electronic data capture system.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 1.1 Billion |
| Market size forecast in 2028 | USD 2.7 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 13.8% from 2022 to 2028 |
| Number of Pages | 272 |
| Number of Tables | 453 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Component, Development Phase, Delivery Mode, End-user, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Based on region, the electronic data capture systems market is segmented into North America, Europe, Asia Pacific, and LAMEA. In 2021, the North American region dominated the electronic data capture systems market by producing the greatest revenue share. This put the region in the position of being the leader. This may be attributed to the highly developed healthcare infrastructure, the presence of significant corporations, the increasing digitization of clinical research, as well as the decentralization of clinical trials. The primary market participants put into practice a variety of tactics with the goal of expanding their market penetration and capabilities.
Free Valuable Insights: Global Electronic Data Capture Systems Market size to reach USD 2.7 Billion by 2028
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Oracle Corporation, IBM Corporation, Veeva Systems, Inc., IQVIA Holdings, Inc., Medidata Solutions, Inc. (Dassault Systems SE), OpenClinica, LLC, DATATRAK International, Inc., Clario, Castor EDC, and Calyx.
By Component
By End-user
By Development Phase
By Delivery Mode
By Geography
The global Electronic Data Capture Systems Market size is expected to reach $2.7 billion by 2028.
Rising demand for eclinical solutions are driving the market in coming years, however, Concerns regarding patient privacy restraints the growth of the market.
Oracle Corporation, IBM Corporation, Veeva Systems, Inc., IQVIA Holdings, Inc., Medidata Solutions, Inc. (Dassault Systems SE), OpenClinica, LLC, DATATRAK International, Inc., Clario, Castor EDC, and Calyx.
The Web & Cloud-based segment is generating highest revenue share in the Global Electronic Data Capture Systems Market by Delivery Mode in 2021 thereby, achieving a market value of $1.8 billion by 2028.
The CROs segment is leading the Global Electronic Data Capture Systems Market by End-user in 2021 thereby, achieving a market value of $931.4 million by 2028.
The North America market dominated the Global Electronic Data Capture Systems Market by Region in 2021 and would continue to be a dominant market till 2028; thereby, achieving a market value of $1.2 billion by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
