
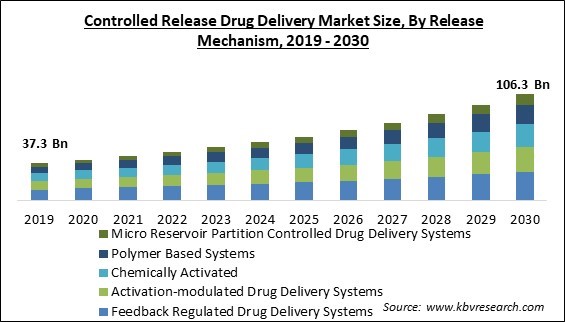
The Global Controlled Release Drug Delivery Market size is expected to reach $106.3 billion by 2030, rising at a market growth of 10.4% CAGR during the forecast period.
The coacervation provides various benefits including improved effectiveness, fewer side effects, convenience, and guaranteed linear medication administration over a predetermined duration, therefore, coacervation segment acquired $5,289.0 million revenue in the market in 2022. Coacervates are a novel family of drug delivery agents created to transport proteins and small-molecule medications. Among these are coacervates made of heparin that hold artificial polycations for the regulated delivery of growth factors. With more people using these devices, they are expected to soon increase in demand for controlled release drug delivery. Some of the factors impacting the market are growing application of non-invasive drug delivery techniques, increase in global aging population proportion, and Challenges in controlled release drug formulation preparation.
Because of technological improvements in medicine and straightforward oral drug delivery methods, the non-invasive mode of targeted medication delivery is now capable of competing with traditional injectables as well as uncomfortable routes of drug delivery. These drug delivery systems are rapidly displacing some of the most common injections given to patients with certain diseases. For example, hypodermic injections are painful, create hazardous medical waste, and raise the risk of disease transmission through reused needles, especially in developing countries. The elderly population is increasing quickly in every nation on the earth. Nearly 1.5 billion people will inhabit this world by 2050, an increase of more than three times the current number. Additionally, during this time, it is predicted that the percentage of elderly citizens will increase to 16.0% worldwide. In the middle of the twenty-first century, one in six people will be 65 or older. The "population aging phenomenon" describes the rise in the proportion and prevalence of older persons globally. Additionally, 703 million people worldwide were 65 or older in 2019. In 2019, there were 9% more persons aged 65 and over than there were in 2018. With the world's aging population growing and these individuals being more susceptible to age-related problems, it is projected that increased demand for novel drug delivery systems will fuel market expansion.
Due to the rising need for solutions for controlled release drug administration, the COVID-19 pandemic had a favorable effect on the market's growth. For instance, the September 2021 publication of an article titled "Role of Novel Drug Delivery Systems in Coronavirus Disease-2019 (COVID-19): Time to Act Now" demonstrated the impending increase in the use and demand for novel drug delivery systems and technologies. This facilitated the release of several novel drug delivery systems and devices, which helped repurpose the existing medications to treat various viral illnesses.
However, drug-related formulation challenges include active pharmaceutical ingredients with short half-lives and the need for higher dose delivery, as well as formulation-related issues. This frequently calls for soluble handling capabilities because most polymers need to be dissolved in natural solvents before being covered by controlled-release formulations (such as tablets, granules, and capsules). Additionally, dumping of doses is a key drawback of controlled release formulation. Dose modification is more challenging in formulations with controlled release. Therefore, the market expansion may be slowed by any technological setbacks or delays.
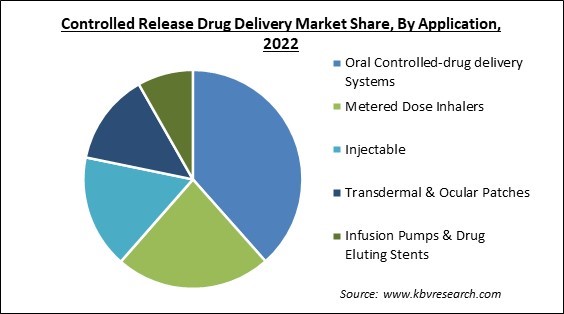
On the basis of application, the controlled release drug delivery market is classified into metered dose inhalers, injectable, transdermal & ocular patches, infusion pumps & drug eluting stents, and oral controlled drug delivery systems. The oral controlled drug delivery systems segment procured the largest revenue share in the controlled release drug delivery market in 2022. By getting beyond obstacles such as low solubility, enzymatic degradation, and first-pass metabolism, oral controlled drug delivery systems can increase the bioavailability of pharmaceuticals. They can speed up the absorption of the medicine and prevent it from degrading in the GI tract, leading to higher and more reliable drug concentrations in the bloodstream. As the drug stays at the target location for a longer period and provides sustained pharmacological activity, this increased drug delivery can improve therapeutic effectiveness.
Based on technology, the controlled release drug delivery market is characterized into Wurster technique, coacervation, micro encapsulation, implants, transdermal, targeted delivery, and others. The micro encapsulation segment procured a considerable growth rate in the controlled release drug delivery market in 2022. Over the projected period, the demand for controlled medication release of extremely complex and unstable compounds, including proteins, antioxidants, and vitamins, is expected to increase. This is projected to support the expansion of the micro encapsulation segment. For example, vitamin A has weak chemical stability and water solubility; nevertheless, when it is micro encapsulated, the shelf life of the product rises, aiding in the achievement of controlled drug release concurrently.
On the basis of release mechanism, the controlled release drug delivery market is classified into polymer-based systems, micro reservoir partition controlled drug delivery systems, feedback regulated drug delivery systems, activation-modulated drug delivery systems, and chemically activated. The feedback regulated drug delivery systems segment recorded the largest revenue share in the controlled release drug delivery market in 2022. Due to its therapeutic effectiveness in treating conditions like diabetes, feedback-regulated medication delivery devices are becoming more popular. Furthermore, it is anticipated that rising R&D efforts to develop feedback-regulated, controlled drug delivery systems as antidotes will support market expansion.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 48.9 Billion |
| Market size forecast in 2030 | USD 106.3 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 10.4% from 2023 to 2030 |
| Number of Pages | 335 |
| Number of Table | 430 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling |
| Segments covered | Release Mechanism, Technology, Application, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region wise, the controlled release drug delivery market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment witnessed the maximum revenue share in the controlled release drug delivery market in 2022. It is projected that increased investment in research and development, in addition to government initiatives that have been carried out to encourage the development of controlled release systems, will drive market expansion in this region. The presence of large market participants is another factor that contributes to the encouragement of the development of controlled release systems. The rise of the demand for these products is helped along by the rising prevalence of chronic ailments such as cancer, diabetes, and heart diseases.
Free Valuable Insights: Global Controlled Release Drug Delivery Market size to reach USD 106.3 Billion by 2030
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Johnson & Johnson, Merck & Co., Inc., Alkermes PLC, Pfizer, Inc., Assertio Holdings, Inc. (Assertio Therapeutics, Inc.), Lonza Group AG (Capsugel), Colorcon, Inc. (BPSI Holdings LLC.), Corium, LLC (Gurnet Point Capital), Adare Pharma Solutions, and Coating Place, Inc.
By Release Mechanism
By Technology
By Application
By Geography
The Market size is projected to reach USD 106.3 billion by 2030.
Increase in global aging population proportion are driving the Market in coming years, however, Challenges in controlled release drug formulation preparation restraints the growth of the Market.
Johnson & Johnson, Merck & Co., Inc., Alkermes PLC, Pfizer, Inc., Assertio Holdings, Inc. (Assertio Therapeutics, Inc.), Lonza Group AG (Capsugel), Colorcon, Inc. (BPSI Holdings LLC.), Corium, LLC (Gurnet Point Capital), Adare Pharma Solutions, and Coating Place, Inc.
The Targeted Delivery segment is leading the Market by Technology in 2022, thereby, achieving a market value of $23.9 billion by 2030.
The Injectable segment has shown the high growth rate of 11.3% during (2023 - 2030).
The North America region dominated the Market by Region in 2022, and would continue to be a dominant market till 2030; thereby, achieving a market value of $42.8 billion by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
