
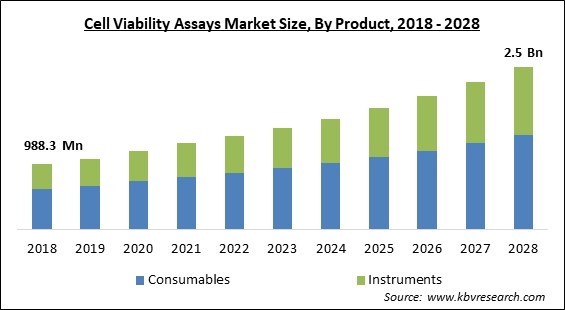
The Global Cell Viability Assays Market size is expected to reach $2.5 billion by 2028, rising at a market growth of 9.7% CAGR during the forecast period.
Cell viability is used to measure the proportion of healthy and live cells in a population, and viability assays are done after the injection of an experimental substance to check the overall health and survival rate of the cells. In addition, cell viability assays are performed to check a drug's efficiency, safety, and therapeutic abilities before it can be launched commercially in the market.
Studies related to viability are also carried out to determine the effectiveness of the cryopreservation techniques, cell culture techniques, and the toxicity of the experimental substance. The assays to measure cellular proliferation, and cytotoxicity are widely utilized to supervise the response of and health of the cultural cells when they are treated with various stimuli.
The type and number of cells with the expected result decide the proper technique for the assay method. The cell proliferation assay is the most suitable option to monitor the number of cellular divisions, DNA synthesis, number of cells, and metabolic activity. Calcein AM and trypan blue are the viability dyes used for cell counting and can also provide both the rate of proliferation and viable cell percentage.
Cell-based assays are generally used for collecting screening of compounds to evaluate if the test molecules have effects on cell proliferation or if they show direct cytotoxic impacts that may lead to cell death. In addition, cell-based assays are often used to measure the binding of receptors and various signal transduction events, which may involve the expression of genetic reporters, monitoring organelle functions, or cellular component’s movement.
From development & support to planning the medicine supply chain, the biotech and pharmaceutical companies are working jointly with the governments to address the COVID-19 pandemic. For example, many commonly used drugs like hydroxychloroquine have seen a sharp rise in the demand for COVID-19 management. The high demand for these drugs has given enormous opportunities for manufacturers of COVID-19 management drugs. In addition, the pharmaceutical and biotechnological companies are expected to see a sharp rise due to the rising demand for vaccines and treatment drugs for novel coronavirus, which will boost the market for cell viability assays.
The increasing amount and size of investments in the healthcare sector by the government of various countries are one of the primary factors boosting the market’s growth. Due to their fast economic development and increasing healthcare cost, many developing nations will have great access to the top-notch healthcare system. The rapid increase of the healthcare sector in various countries can be assed by the rising increase in per-capita spending on healthcare and rising demand for quality and affordable healthcare services. With the rising healthcare sector and increasing funding, market for cell viability assays will propel.
The cases of chronic diseases are increasing owing to the changing lifestyles and the increasing old age population, increasing obesity levels, easy & broad availability of tobacco, and urbanization. According to the United Nations, every three out of five deaths are related to four major non-communicable diseases (NCDs), which are diabetes, cardiovascular disease, chronic lung disease, and cancer. The management and prevention of NCDs have become a priority globally due to the rising burden of NCDs, especially in low-income countries. The growing incidences and frequency of infectious and chronic diseases are aiding the cell viability assay market to develop.
The shortage of healthcare professionals is due to the investments in the training and education of healthcare workers in many countries. Also, the mismatch in the employment and education policies concerning the healthcare system and the population requirement is a significant factor. The situation worsens due to difficulties deploying healthcare workers in remote, underserved, and rural areas. Additionally, there is an increase in the migration of health workers, which may further increase the shortage, especially in low and lower-middle-income nations.
Based on the product, the cell viability assays market is segmented into consumables and instrument. Because pharmaceutical companies perform research & development with instruments like liquid chromatographers, nuclear resonance (NMR) spectrometers and gas chromatographer, which significantly increase the development and research cycles, and bring beneficial drugs to the market at a faster rate. Instrumental methods for the analysis of the cell viability gives significant advantages over manual ones.
On the basis of application, the cell viability assays market is classified into drug discovery & development, stem cell research and diagnostics. The drug discovery & development segment recorded a considerable growth rate in the cell viability assays market in 2021. This is because the cell viability assay plays an essential role in various stages of drug development and discovery because of its adaptability, versatility, and flexibility. Cell viability is primarily used to assess the response of the cells against a drug or a chemical compound during the drug discovery and development process.
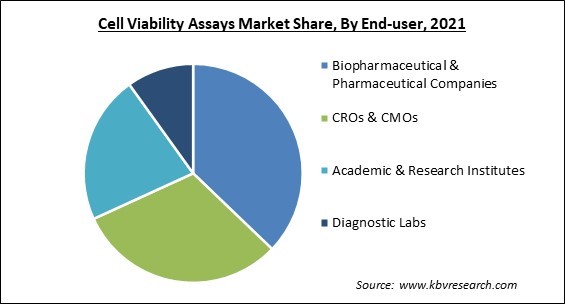
By end user, the cell viability assays market is bifurcated into Biopharmaceutical & Pharmaceutical Companies, CROs & CMOs, Academic & Research Institutes and Diagnostic Labs. The biopharmaceutical & pharmaceutical companies segment procured the highest growth rate in the cell viability assays market in 2021. This is attributable from the increasing usage of viability assays in pharmaceuticals to assess the influence of the developed agents’ cells. Researchers employ various cells to determine the effect of the developed treatment, which often targets cancer tumors.
| Report Attribute | Details |
|---|---|
| Market size value in 2021 | USD 1.3 Billion |
| Market size forecast in 2028 | USD 2.5 Billion |
| Base Year | 2021 |
| Historical Period | 2018 to 2020 |
| Forecast Period | 2022 to 2028 |
| Revenue Growth Rate | CAGR of 9.7% from 2022 to 2028 |
| Number of Pages | 355 |
| Number of Tables | 643 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Competitive Landscape, Companies Strategic Developments, Company Profiling |
| Segments covered | Product, Application, End-user, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Region-wise, the cell viability assays market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region led the cell viability assays market by generating maximum revenue share in 2021. The market is expanding in this region because of the introduction of various government schemes, the rising cases of chronic diseases like cancer, and the presence of high-quality infrastructure for laboratory and clinical research in the region. There is also a lean towards stem cell-based research, which further opens new treatment opportunities.
Free Valuable Insights: Global Cell Viability Assays Market size to reach USD 2.5 Billion by 2028
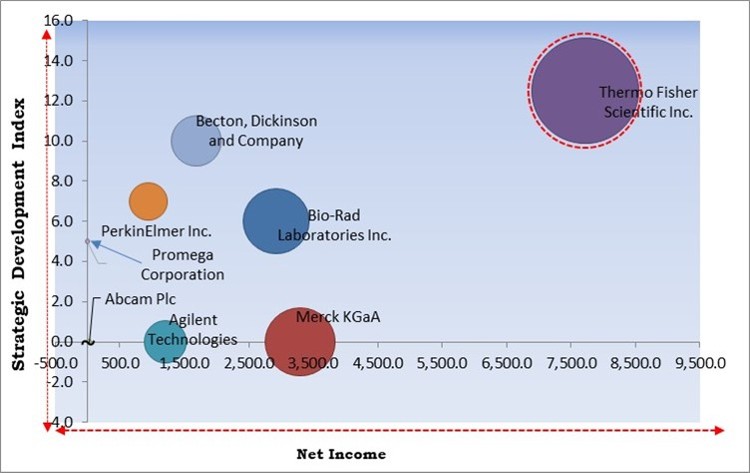
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; Thermo Fisher Scientific, Inc. is the forerunner in the Cell Viability Assays Market. Companies such as Becton, Dickinson and Company, Merck KGaA, Bio-Rad Laboratories, Inc. are some of the key innovators in Cell Viability Assays Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Thermo Fisher Scientific Inc., Agilent Technologies, Inc., Bio-Rad Laboratories Inc., Merck KGaA, Becton, Dickinson and Company, PerkinElmer Inc., Promega Corporation, Biotium, Creative Bioarray, and Abcam Plc.
By Product
By End User
By Application
By Geography
The global Cell Viability Assays Market size is expected to reach $2.5 billion by 2028.
Rising Cases Of Infectious And Chronic Disease are driving the market in coming years, however, The Unavailability Of Healthcare Professionals restraints the growth of the market.
Thermo Fisher Scientific Inc., Agilent Technologies, Inc., Bio-Rad Laboratories Inc., Merck KGaA, Becton, Dickinson and Company, PerkinElmer Inc., Promega Corporation, Biotium, Creative Bioarray, and Abcam Plc.
The Consumables segment acquired maximum revenue share in the Global Cell Viability Assays Market by Product in 2021 thereby, achieving a market value of $1.4 billion by 2028.
The Stem Cell Research segment is leading the Global Cell Viability Assays Market by Application in 2021 thereby, achieving a market value of $1 billion by 2028.
The North America market dominated the Global Cell Viability Assays Market by Region in 2021, and would continue to be a dominant market till 2028; thereby, achieving a market value of $879.6 million by 2028.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
