
The Global Autoinjectors Market size is expected to reach $5.3 billion by 2026, rising at a market growth of 17.6% CAGR during the forecast period. An autoinjector is a medical device consist of a syringe having a spring-charged needle containing a pre-charged dosage of drugs and is designed to administer a dose of a specific drug. These injectors were primarily designed to overcome the fear related to the needle-based drug delivery device used for self-administration.
The system activates and administers a measured amount of a drug when pushed into the body of the subject with a downward motion. Autoinjectors are extensively used for self-administration of epinephrine in order to prevent anaphylaxis. Epinephrine is taken by migraine sufferers to get immediate pain relief and also for other medical emergency treatments. Auto-injectors have multiple benefits including reducing needle-related phobia disorder, decreases the risks of needle jammed accidents, make sure dosage quality steadiness, and serving increase effectiveness.
The auto-injector market is likely to register considerable growth in the coming years, due to increased cases of anaphylaxis. In addition to it, there is a rapid rise in R&D efforts by manufacturing companies. These efforts are in direction to develop simple, cost-effective, and novel technology-based autoinjectors that can be used for the treatment of chronic diseases including rheumatoid arthritis and multiple sclerosis. Such developments are anticipated to drive the market growth during the forecast period.
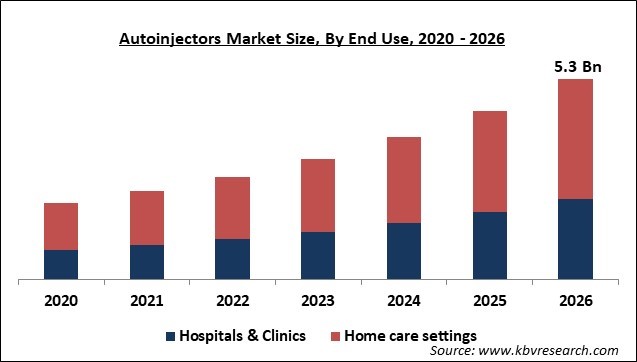
Based on End Use, the market is segmented into Hospitals & Clinics and Home care settings. The hospital segment is expected to grow at faster pace in the coming years due to a surge in the number of patients who are suffering from multiple sclerosis and anaphylaxis. Multiple sclerosis is very common in the U.S., Europe, Canada, and New Zealand. It affects more than 400,000 people alone in the U.S. and about 2.5 million people all across the globe.
Based on Type, the market is segmented into Disposable autoinjectors and Reusable autoinjectors. The disposable autoinjectors segment had the largest share of the global market of autoinjectors in 2019. Disposable autoinjectors are preferred mostly owing to their ease of use and also the presence of the built-in glass syringe eliminates the necessity of manually filling the glass syringe, which makes it more suitable for patients with visual impairments or reduced dexterity.
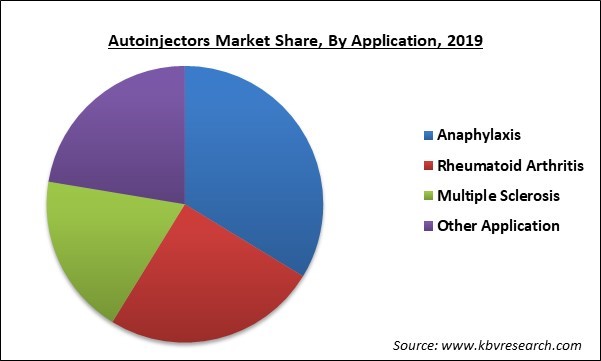
Based on Application, the market is segmented into Anaphylaxis, Rheumatoid Arthritis, Multiple Sclerosis and other Applications. In 2019, Anaphylaxis segment had the largest share of the autoinjectors market. This is mainly accredited to its high occurrence. The growing incidence of rheumatoid arthritis also drives the demand for autoinjectors worldwide. As per the CDC, around 43.7 million adults which comprise 22.7% of the total population of the US are affected by some form of arthritis such as RA, lupus, gout, or fibromyalgia annually.
| Report Attribute | Details |
|---|---|
| Market size value in 2019 | USD 1.7 Billion |
| Market size forecast in 2026 | USD 5.3 Billion |
| Base Year | 2019 |
| Historical Period | 2016 to 2018 |
| Forecast Period | 2020 to 2026 |
| Revenue Growth Rate | CAGR of 17.6% from 2020 to 2026 |
| Number of Pages | 205 |
| Number of Tables | 343 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Companies Strategic Developments, Company Profiling, Competitive Landscape |
| Segments covered | Type, Application, End Use, Region |
| Country scope | US, Canada, Mexico, Germany, UK, France, Russia, Spain, Italy, China, Japan, India, South Korea, Singapore, Malaysia, Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria |
| Growth Drivers |
|
| Restraints |
|
Free Valuable Insights: Global Autoinjectors Market to reach a market size of $5.3 Billion by 2026
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. The growth of the market in the Asia Pacific market is largely due to the large number of patients suffering from chronic allergies and diabetes as well as increasing healthcare expenditure. This in turn has drawn a number of key auto-injector device manufacturers to this region. These companies are showing more presence in the Asia Pacific market via several approaches, including partnering with local pharmaceutical companies and establishing sales offices. The growing occurrence of type 1 diabetes is expected to drive market growth.
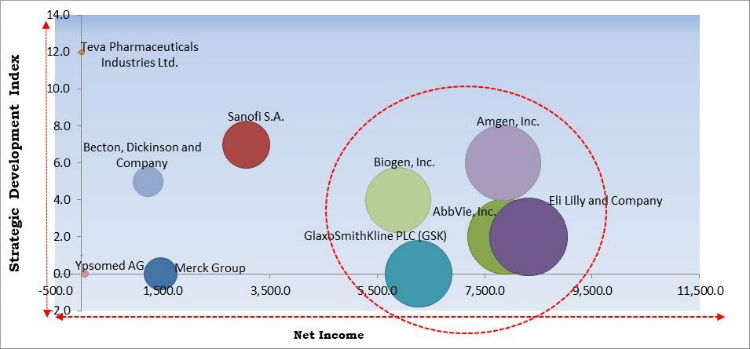
The major strategies followed by the market participants are Product Launches and Partnerships. Based on the Analysis presented in the Cardinal matrix; Amgen, Inc., Biogen, Inc., and GlaxoSmithKline PLC, Company are the forerunners in the Auto-Injectors Market. Companies such as Sanofi S.A., Teva Pharmaceuticals Industries Ltd., Becton, Dickinson and Company, Ypsomed AG, and Merck Group are some of the key innovators in the market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Eli Lilly and Company, GlaxoSmithKline PLC (GSK), Merck Group, Sanofi S.A., Becton, Dickinson and Company, Teva Pharmaceuticals Industries Ltd., Amgen, Inc., Ypsomed AG, AbbVie, Inc. and Biogen, Inc.
By Type
By Application
By End Use
By Geography
Companies Profiled
The autoinjectors market size is projected to reach USD 5.3 billion by 2026.
The major factors that are anticipated to drive the autoinjectors industry include increasing prevalence of anaphylaxis.
The home care settings segment had the largest market share in the year 2019 and can largely be credited to the swift growth in the geriatric population in the world and the increasing requirement for cost-effective administration of drugs.
Eli Lilly and Company, GlaxoSmithKline PLC (GSK), Merck Group, Sanofi S.A., Becton, Dickinson and Company, Teva Pharmaceuticals Industries Ltd., Amgen, Inc., Ypsomed AG, AbbVie, Inc. and Biogen, Inc.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
