
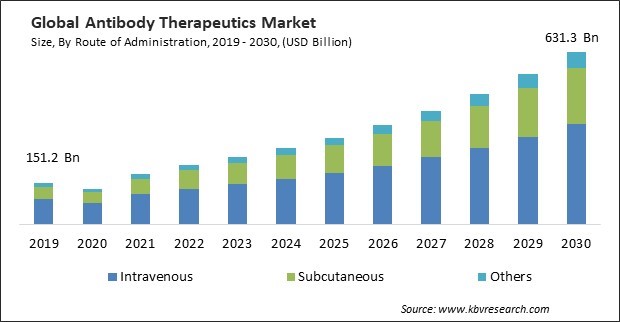
The Global Antibody Therapeutics Market size is expected to reach $631.3 billion by 2030, rising at a market growth of 14.4% CAGR during the forecast period.
Antibody therapeutics are explored to prevent and treat respiratory infections, like those caused by respiratory syncytial virus (RSV), influenza, and other respiratory pathogens. Thus, the Infectious Diseases segment acquired $27,652.5 million in 2022. Monoclonal antibodies can provide passive immunity and reduce the risk of severe disease. It offers a form of passive immunization, providing immediate protection against infectious agents. This is particularly important in situations where there is a need for rapid intervention, such as in the case of post-exposure prophylaxis. Some of the factors impacting the market are technological advancements in antibody engineering, increasing number of senior citizens and high development costs of antibody therapeutics.
Advances in antibody engineering technologies, including antibody-drug conjugates (ADCs), Fc engineering, and next-generation sequencing, enhance the properties and performance of antibody therapeutics. These technological advancements contribute to developing more potent, specific, and well-tolerated antibody drugs. Single-cell antibody technologies enable the isolation and characterization of individual B cells, allowing for the identification of rare and high-affinity antibodies. This approach is particularly valuable when traditional hybridoma or phage display methods may not capture the full diversity of the immune response. Single-cell antibody technologies contribute to developing antibodies with enhanced specificity and functionality. Additionally, the aging process is associated with an elevated risk of various age-related diseases, including cancer, neurodegenerative disorders, and autoimmune diseases. Antibody therapeutics, with their targeted and precision-based mechanisms, are well-suited for addressing the underlying causes of these diseases. As per the World Health Organization (WHO), one in six people will be 60 or older by 2030. The number of people 60 and older in the world will increase from 1 billion in 2020 to 1.4 billion in 2050. By 2050, there will be 2.1 billion people in the world who are 60 or older. It is anticipated that there will be 426 million persons 80 or older by 2050. This preference for targeted therapies aligns with the growing awareness of minimizing side effects in older populations. Increasing number of senior citizens has been a pivotal factor in driving the growth of the market.
Further, the pandemic highlighted vulnerabilities in global supply chains, affecting pharmaceutical product development and distribution, particularly antibody therapeutics. Disruptions in the supply chain, shortages of critical raw materials, and logistical challenges impacted the manufacturing and delivery of antibody drugs. Monoclonal antibodies were also deployed for prophylactic use in individuals at high risk of exposure to COVID-19, like those with underlying health conditions or individuals in close contact with infected individuals. The success of mRNA vaccines, such as those developed by Pfizer-BioNTech and Moderna, shifted attention towards nucleic acid-based therapies. While there was a heightened focus on infectious disease research, including antibody therapeutics for COVID-19, other therapeutic areas experienced shifts in priorities and resource allocation. Thus, the COVID-19 pandemic had a moderate effect on the market.
However, the research and development of antibody therapeutics involves substantial costs, including preclinical studies, clinical trials, and manufacturing expenses. The high investment required can pose a barrier, especially for smaller biotech companies, hindering their ability to bring novel antibodies to market. The financial burden associated with antibody development may lead to a focus on well-established targets and therapeutic areas, potentially limiting exploration in areas with significant unmet medical needs. This reduced innovation and diversity in the development pipeline can hinder the discovery of breakthrough therapies. High development costs of antibody therapeutics are a significant challenge that hampers the growth of the market.
On the basis of route of administration, the market is divided into intravenous, subcutaneous, and others. In 2022, the intravenous segment dominated the market with maximum revenue share. Intravenous administration ensures rapid and complete bioavailability of antibody therapeutics. This route allows the drug to enter the bloodstream directly, avoiding barriers such as the gastrointestinal tract, which can impact absorption rates. Intravenous delivery allows for precise dosing and control over the rate of administration. Healthcare professionals can carefully regulate the infusion rate to achieve therapeutic levels in the bloodstream, optimizing the efficacy of antibody therapeutics. Intravenous administration provides an immediate onset of action for antibody therapeutics. This is crucial when rapid therapeutic effects are needed, such as in treating acute conditions or emergencies.
By format, the market is categorized into monoclonal antibody, polyclonal antibody therapy, bispecific antibody, antibody fragment, and others. The antibody fragment segment procured a promising growth rate in the market in 2022. Antibody fragments typically exhibit faster clearance from the circulatory system compared to full-length antibodies. This rapid clearance can be beneficial when a quick onset of action or a shorter half-life is desired, providing greater control over the timing and duration of the therapeutic effect. Antibody fragments are widely used in diagnostic imaging applications. Their smaller size allows for rapid clearance from non-target tissues, improving imaging contrast. Single-photon emission computed tomography (SPECT) and positron emission tomography (PET) frequently use radiolabeled antibody fragments. Antibody fragments are explored for therapeutic applications in autoimmune diseases, where targeted and localized immunosuppression is desired.
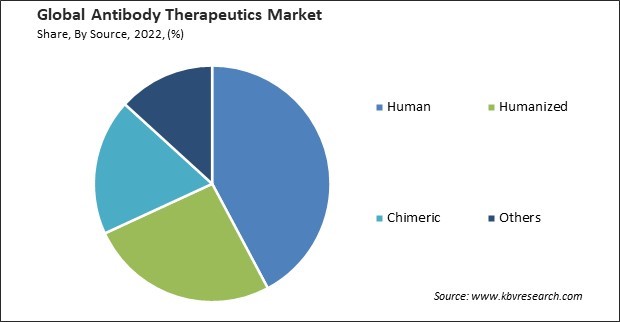
By source, the market is segmented into human, humanized, chimeric, and others. In 2022, the human segment registered the maximum revenue share in the market. Human innovation and technological advancements, such as genetic engineering, monoclonal antibody production, and novel drug delivery systems, contribute to creating more effective and targeted antibody therapies. Human efforts in educating healthcare professionals, patients, and the public about the benefits and availability of antibody therapeutics contribute to their acceptance and utilization. Humans participate in clinical trials to test the efficacy and safety of antibody therapeutics. Their participation generates valuable data that inform the development process and regulatory approvals.
Based on end user, the market is fragmented into hospitals, long-term care facilities, and others. The long-term care facilities segment covered a considerable revenue share in the market in 2022. Long-term care facilities often cater to an aging population, which is more susceptible to conditions that may benefit from antibody therapeutics, including certain types of cancer, rheumatoid arthritis, and neurodegenerative disorders. The use of antibody therapeutics in long-term care facilities can reduce hospitalization rates. This is particularly important for residents who may have difficulty traveling to hospitals or are at higher risk of complications from hospital stays. Antibody therapeutics, particularly monoclonal antibodies, can prevent and manage infections. These treatments can support overall health maintenance in long-term care settings where people may be more susceptible to infections.
Based on disease areas, the market is classified into autoimmune & inflammatory diseases, oncology, hematology, infectious diseases, osteology, immunology, neurology, and others. The oncology segment procured a promising growth rate in the market in 2022. Antibody therapeutics are often combined with other cancer treatments, such as chemotherapy, targeted therapies, and radiation therapy. Combinatorial approaches aim to maximize treatment efficacy and address multiple aspects of cancer biology. Antibody therapeutics have proven effective in treating hematologic cancers, including various lymphomas and leukemias. Monoclonal antibodies, such as rituximab and alemtuzumab, target specific markers on cancer cells, leading to improved outcomes. Radioimmunotherapy involves antibodies labeled with radioactive isotopes to selectively deliver radiation to cancer cells. Ibritumomab, tiuxetan, and tositumomab are examples of radioimmunotherapy used in certain lymphomas.
| Report Attribute | Details |
|---|---|
| Market size value in 2022 | USD 216.8 Billion |
| Market size forecast in 2030 | USD 631.3 Billion |
| Base Year | 2022 |
| Historical Period | 2019 to 2021 |
| Forecast Period | 2023 to 2030 |
| Revenue Growth Rate | CAGR of 14.4% from 2023 to 2030 |
| Number of Pages | 425 |
| Number of Table | 600 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Porter’s 5 Forces Analysis, Company Profiling, Companies Strategic Developments, SWOT Analysis, Winning Imperatives |
| Segments covered | Route of Administration, Format, Source, End User, Disease Areas, Region |
| Country scope |
|
| Companies Included | F. Hoffmann-La Roche Ltd, AbbVie, Inc., Johnson & Johnson (Johnson & Johnson Services, Inc.), Merck KGaA, Bristol Myers Squibb Company, AstraZeneca PLC, Sanofi S.A., Novartis AG, Biogen, Inc. and Amgen, Inc. |
| Growth Drivers |
|
| Restraints |
|
Region-wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. In 2022, the North America region led the market by generating the highest revenue share. North America, particularly the United States, is home to a vibrant and well-established pharmaceutical research and development ecosystem. North America is a global hub for clinical trials, providing a robust infrastructure for conducting large-scale and diverse studies. The North American population has a relatively high prevalence of autoimmune diseases, like rheumatoid arthritis, psoriasis, and inflammatory bowel diseases.
Free Valuable Insights: Global Antibody Therapeutics Market size to reach USD 631.3 Billion by 2030
The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include F. Hoffmann-La Roche Ltd, AbbVie, Inc., Johnson & Johnson (Johnson & Johnson Services, Inc.), Merck KGaA, Bristol Myers Squibb Company, AstraZeneca PLC, Sanofi S.A., Novartis AG, Biogen, Inc. and Amgen, Inc.
By Route of Administration
By Format
By Source
By End User
By Disease Areas
By Geography
The Market size is projected to reach USD $631.3 billion by 2030.
Technological advancements in antibody engineering are driving the Market in coming years, however, High development costs of antibody therapeutics restraints the growth of the Market.
F. Hoffmann-La Roche Ltd, AbbVie, Inc., Johnson & Johnson (Johnson & Johnson Services, Inc.), Merck KGaA, Bristol Myers Squibb Company, AstraZeneca PLC, Sanofi S.A., Novartis AG, Biogen, Inc. and Amgen, Inc.
The Monoclonal Antibody segmenat is generating the highest revenue in the Market by Format in 2022; thereby, achieving a market value of $451.1 billion by 2030.
The Hospitals segment is leading the Market by End User in 2022; thereby, achieving a market value of $360.5 billion by 2030.
The North America region dominated the Market by Region in 2022 and would continue to be a dominant market till 2030; thereby, achieving a market value of $231.6 billion by 2030.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.
