According to a new report, published by KBV research, The Global Regulatory Information Management System Market size is expected to reach $4.7 billion by 2031, rising at a market growth of 10.5% CAGR during the forecast period.
RIMS provide real-time visibility into regulatory activities, compliance status, and performance metrics through customizable dashboards and reports. They allow stakeholders to monitor important indicators, measure progress, and make informed choices based on actionable insights. RIMS automates repetitive tasks, reduces manual paperwork, and streamlines regulatory processes, improving productivity and efficiency. They enable staff to focus on value-added activities, such as regulatory strategy development, rather than administrative tasks, leading to cost savings and resource optimization.
The Medical Device Sector segment is experiencing a CAGR of 11.1% during (2024 - 2031). Medical device companies deal with sensitive patient data and confidential information. RIMS provides strong data security features such as encryption, access controls, and audit trails to secure regulated information from unauthorized access or tampering. They also provide traceability of regulatory activities, ensuring accountability and transparency in regulatory processes. Medical devices undergo multiple stages, from design and development to post-market surveillance. RIMS support lifecycle management by providing tools for tracking regulatory changes, managing product registrations, and ensuring compliance with evolving regulations throughout the product lifecycle.
Full Report: https://www.kbvresearch.com/regulatory-information-management-system-market/
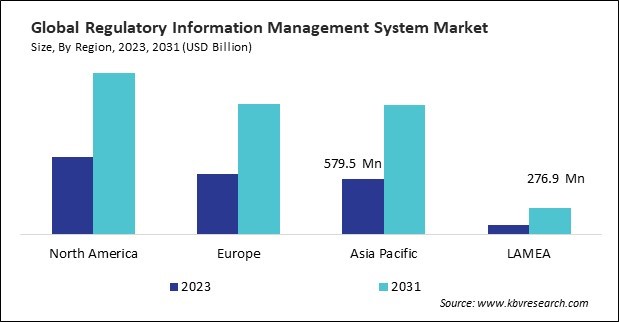
The North America region dominated the Global Regulatory Information Management System Market by Region in 2023; thereby, achieving a market value of $1.7 billion by 2031. The Europe region is anticipating a CAGR of 10.1% during (2024 - 2031). Additionally, The Asia Pacific region would exhibit a CAGR of 11.3% during (2024 - 2031).
By End User
 Unique Offerings
Unique Offerings