According to a new report, published by KBV research, The Global Bioprocess Validation Market size is expected to reach USD 994 billion by 2032, rising at a market growth of 8.8% CAGR during the forecast period.
Competition varies by region. In mature markets (North America, Europe), competition is fierce and saturated; providers compete on differentiation, speed, and cost. In emerging markets, there is room for local, regional entrants to capture business—particularly where customers prefer local validators familiar with local regulatory regimes.
The In house segment is experiencing a CAGR of 8.5 % during the forecast period. This strong preference for internal validation reflects the need for greater control over critical processes, intellectual property protection, and compliance with strict regulatory requirements. Companies conducting in-house validation benefit from direct oversight of testing activities, streamlined communication between R&D and manufacturing teams, and faster turnaround times.
The Continued Process Verification segment led the maximum revenue in the Global Bioprocess Validation Market by Stage in 2024, thereby, achieving a market value of USD 389.2 million by 2032. This dominance reflects the increasing regulatory emphasis on ongoing monitoring to ensure that processes remain in a state of control throughout the product lifecycle. Continued verification enables manufacturers to identify deviations in real-time, maintain product quality, and reduce compliance risks.
The Bioprocess Residuals Testing segment is growing at a CAGR of 7.8 % during the forecast period. This dominance is largely due to the critical importance of detecting and quantifying impurities that arise during the manufacturing of biologics, such as host cell proteins, DNA fragments, endotoxins, and other process-related contaminants. Regulatory bodies impose strict guidelines to ensure that these impurities are identified and effectively removed, as even trace amounts can compromise the safety and efficacy of biopharmaceutical products.
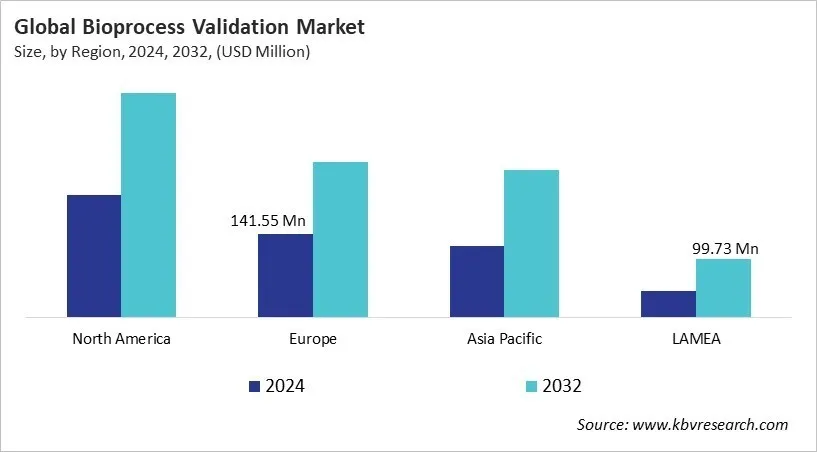
The North America region dominated the Global Bioprocess Validation Market by Region in 2024, thereby, achieving a market value of USD 380.3 million by 2032. The Europe region is anticipated to grow a CAGR of 8.4% during (2025 - 2032). Additionally, The Asia Pacific region would witness a CAGR of 9.7% during (2025 - 2032).
By Mode
By Stage
By Testing Type
By Geography
 Unique Offerings
Unique Offerings