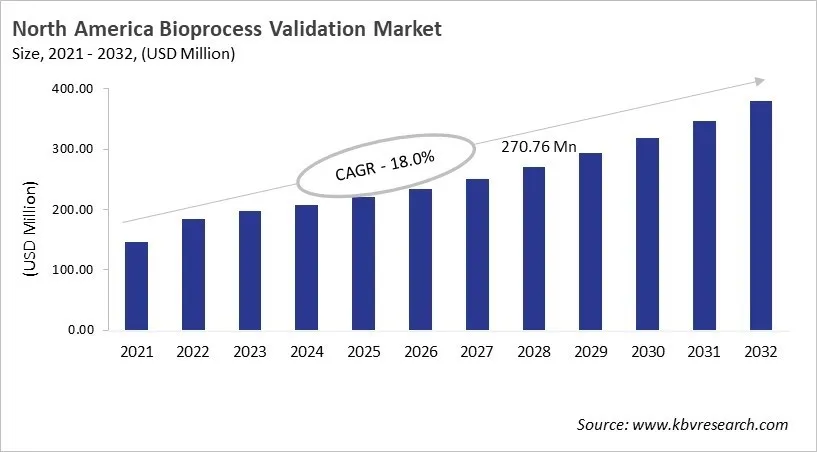
The North America Bioprocess Validation Market would witness market growth of 8.1% CAGR during the forecast period (2025-2032).
The US market dominated the North America Bioprocess Validation Market by Country in 2024, and would continue to be a dominant market till 2032; thereby, achieving a market value of USD 264.2 million by 2032. The Canada market is experiencing a CAGR of 9.9% during (2025 - 2032). Additionally, The Mexico market would exhibit a CAGR of 9.6% during (2025 - 2032). The US and Canada led the North America Bioprocess Validation Market by Country with a market share of 73.4% and 15% in 2024.
The North American bioprocess validation market has changed from basic end-point checks to a full discipline based on the lifecycle that supports modern biopharmaceutical manufacturing. Early validation was mostly about making sure that equipment was safe to use. But as biologics, advanced vaccines, and recombinant therapies became more popular, regulatory agencies started to require ongoing monitoring and compliance at the design stage. New validation methods were needed to check the integrity and compatibility of new technologies like single-use systems, disposable bioreactor bags, and continuous manufacturing. Digital platforms and process analytical technologies now make it possible to collect data in real time. This turns validation into an ongoing process that makes sure products are reliable and can be released more quickly. Outsourcing has grown a lot, and specialized service providers help biopharma companies stay compliant with regulations while focusing on their core research and development.
Three big changes in the market right now are the use of real-time, data-driven validation, the need for extensive validation of single-use and modular systems, and the growing reliance on specialized outsourcing. Companies spend a lot of money on digital tools, automated testing, and predictive analytics to speed up processes and make better decisions. Top companies package equipment with validation services, move into biotech hubs, and make plans for new therapies like cell and gene therapies. Global equipment suppliers with integrated solutions, independent validation firms that offer unbiased expertise, and regional players that offer flexible, affordable services all shape the competition. A moderately consolidated but very dynamic market landscape is necessary for success. This is because regulatory credibility, strong documentation, and new ideas in automation and analytics are all important.
Based on Testing Type, the market is segmented into Bioprocess Residuals Testing, Extractables & Leachables Testing, Viral Clearance Testing, Wireless and IoT Penetration Testing, and Other Testing Type. Among various US Bioprocess Validation Market by Testing Type; The Bioprocess Residuals Testing market achieved a market size of USD $42.6 Million in 2024 and is expected to grow at a CAGR of 6.4 % during the forecast period. The Viral Clearance Testing market is predicted to experience a CAGR of 8% throughout the forecast period from (2025 - 2032).
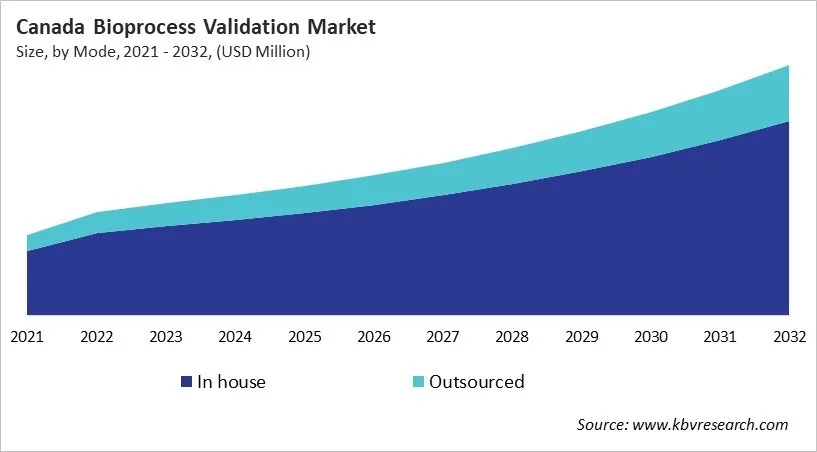
Based on Mode, the market is segmented into In house and Outsourced. The In house market segment dominated the Canada Bioprocess Validation Market by Mode is expected to grow at a CAGR of 9.6 % during the forecast period thereby continuing its dominance until 2032. Also, The Outsourced market is anticipated to grow as a CAGR of 11.2 % during the forecast period during (2025 - 2032).
Free Valuable Insights: The Bioprocess Validation Market is Predict to reach USD 994 Million by 2032, at a CAGR of 8.8%
The US is the leader in the bioprocess validation market because it has a strong biopharmaceutical industry, strict FDA rules, and a lot of money goes into research and development. The need for accurate, fully validated manufacturing processes is growing because more people want biologics, biosimilars, and advanced therapies like cell and gene treatments. Market trends show that more and more people are using single-use technologies, modular systems, automation, and real-time data analytics to make systems more scalable, lower the risk of contamination, and allow for predictive maintenance. There is a lot of competition, with big companies like Thermo Fisher, Danaher, Merck KGaA, Sartorius, and Lonza, as well as many CDMOs that offer specialized validation services. To stay ahead of the competition, top companies are adding more services, using more advanced analytical tools, and working with schools and businesses. Market growth will continue because of ongoing regulatory pressure and the move toward more efficient and flexible manufacturing.
By Mode
By Stage
By Testing Type
By Country
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

