“Global Monoclonal Antibodies In Veterinary Health Market to reach a market value of USD 4.33 Billion by 2032 growing at a CAGR of 18.5%”
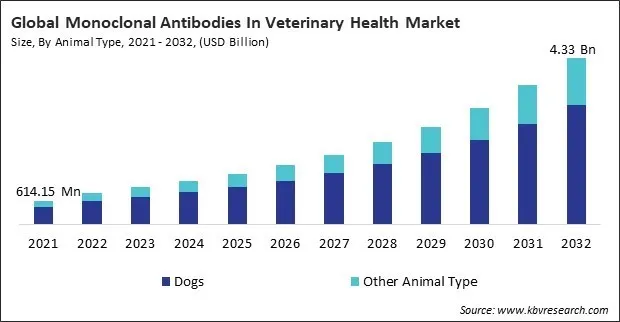
The Global Monoclonal Antibodies In Veterinary Health Market size is expected to reach $4.33 billion by 2032, rising at a market growth of 18.5% CAGR during the forecast period.
Dogs represent one of the most significant animal segments in the Monoclonal Antibodies (mAbs) in Veterinary Health Market due to their widespread adoption, close human companionship, and increasing demand for high-quality pet healthcare. Companion animals, particularly dogs, experience chronic diseases, cancer, allergies, and infections—conditions that can now be effectively targeted by monoclonal antibody therapies.
The onset of the COVID-19 pandemic brought the global sports ecosystem to a near standstill. Live sporting events were canceled or postponed indefinitely, significantly reducing opportunities for AI deployment in areas such as performance analytics, player tracking, and fan engagement. Without real-time data from ongoing games, AI systems that depended on continuous data inflow lost operational relevance and accuracy. During the pandemic, economic uncertainty and shifting financial priorities forced many sports organizations and tech firms to cut back on research and development initiatives. AI projects, particularly those still in their pilot phases, were either paused or shelved. Thus, the COVID-19 pandemic had a negative impact on the market.
The increasing prevalence of zoonotic and chronic diseases among animals stands as a primary driver of the monoclonal antibodies (mAbs) market in veterinary health. Zoonotic diseases—those transmissible from animals to humans—pose significant public health risks, particularly in regions where human-animal interactions are high, such as agricultural zones and developing nations with close livestock contact. Examples include rabies, brucellosis, leptospirosis, and bovine tuberculosis. In conclusion, the rising incidence of zoonotic and chronic diseases creates a strong and urgent need for effective, targeted therapies, and monoclonal antibodies meet this need with precision, safety, and long-term benefits, positioning them as essential tools in modern veterinary medicine.
Additionally, Technological innovations in antibody discovery, engineering, and biomanufacturing have significantly lowered the barriers to developing veterinary monoclonal antibodies. Earlier, the cost-intensive and technically demanding process of antibody generation limited their use to human healthcare. However, the rapid evolution of hybridoma technology, phage display libraries, and recombinant DNA platforms now allows for high-throughput screening and precision engineering of species-specific antibodies tailored to veterinary needs. Thus, the synergy of bioengineering, manufacturing innovations, and digital platforms has transformed monoclonal antibody development from a theoretical option to a practical and scalable solution for animal health, propelling market expansion.
The development and deployment of monoclonal antibodies in veterinary health are significantly hindered by high research and production costs. Developing a monoclonal antibody involves advanced biotechnological processes, rigorous testing protocols, and substantial investment in infrastructure. Unlike conventional small-molecule drugs, which can often be synthesized through well-established chemical pathways, mAbs require living cell cultures (often Chinese Hamster Ovary cells) and intricate purification methods. In conclusion, the high cost of monoclonal antibody development, regulatory approval, and market delivery acts as a primary restraint on the adoption of this technology in veterinary health.
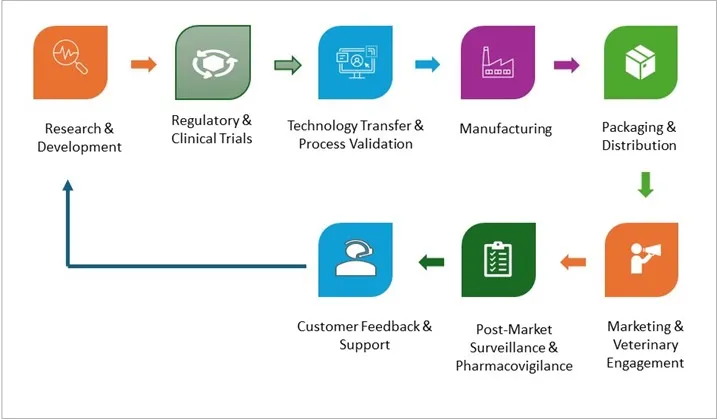
The value chain of the Monoclonal Antibodies in Veterinary Health Market begins with Research & Development, focusing on scientific innovation to create effective antibody therapies. This is followed by Regulatory & Clinical Trials, ensuring safety, efficacy, and compliance. The Technology Transfer & Process Validation phase establishes scalable, quality-assured production processes, leading to Manufacturing of the final products. Afterward, Packaging & Distribution ensures safe delivery to markets. Marketing & Veterinary Engagement promotes awareness among veterinarians and end-users. Post-launch, Post-Market Surveillance & Pharmacovigilance monitor safety, while Customer Feedback & Support provide valuable insights to drive future improvements and innovation.
Based on animal type, the monoclonal antibodies in veterinary health market is characterized into dogs and others. The others segment procured 26% revenue share in the monoclonal antibodies in veterinary health market in 2024. This segment comprises animals such as cats, horses, and certain livestock species. While its share in the monoclonal antibodies market is smaller, this segment is gradually gaining attention as research extends to new species. There is increasing interest in exploring the benefits of monoclonal antibodies for feline diseases, equine inflammatory conditions, and preventive health in farm animals.
| Category | Details |
|---|---|
| Use Case Title | Confidential |
| Date | 2025 |
| Entities Involved | Confidential |
| Objective | To harness monoclonal antibody therapies for controlling infectious diseases and immunological disorders in non-canine animals such as cattle, swine, and zoo animals, enhancing herd health and biosecurity. |
| Context and Background | While mAb usage began in the companion animal sector, growing demand for residue-free therapeutics and antimicrobial alternatives has driven interest in biologics for livestock and exotic species. In 2025, global veterinary authorities promoted mAb research to address zoonotic threats and reduce antibiotic use in the food chain. |
| Description | Innovations in recombinant protein engineering and expression systems enabled scalable production of monoclonal antibodies for larger animals. Applications include:
|
| Key Capabilities Deployed |
|
| Benefits |
|
| Source | Confidential |
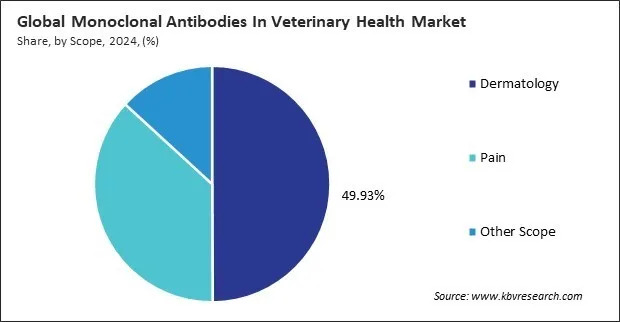
On the basis of scope, the monoclonal antibodies in veterinary health market is classified into dermatology, pain, and others. The pain segment recorded 37% revenue share in the monoclonal antibodies in veterinary health market in 2024. The pain segment plays a vital role in the veterinary monoclonal antibodies space, focusing on the management of chronic and acute pain, especially in conditions like osteoarthritis. Monoclonal antibodies designed for pain relief work by targeting nerve growth factors or other pain-related pathways, providing sustained and non-opioid-based solutions for animal discomfort.
By end user, the monoclonal antibodies in veterinary health market is divided into veterinary hospitals and others. The others segment garnered 22% revenue share in the monoclonal antibodies in veterinary health market in 2024. The others segment includes independent veterinary clinics, mobile veterinary services, and in some cases, academic or research institutions. While this category accounts for a smaller share of the market, it plays a vital role in extending the accessibility of monoclonal antibody treatments beyond large hospital settings.
Free Valuable Insights: Global Monoclonal Antibodies In Veterinary Health Market size to reach USD 4.33 Billion by 2032
Region-wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment recorded 42% revenue share in the monoclonal antibodies in veterinary health market in 2024. North America represents the leading regional market for monoclonal antibodies in veterinary health. The region benefits from a well-established veterinary healthcare infrastructure, widespread adoption of advanced biologic therapies, and high pet ownership rates. Regulatory support and the presence of key pharmaceutical players further enhance the development and commercialization of monoclonal antibodies.
| Report Attribute | Details |
|---|---|
| Market size value in 2024 | USD 1.13 Billion |
| Market size forecast in 2032 | USD 4.33 Billion |
| Base Year | 2024 |
| Historical Period | 2021 to 2023 |
| Forecast Period | 2025 to 2032 |
| Revenue Growth Rate | CAGR of 18.5% from 2025 to 2032 |
| Number of Pages | 309 |
| Number of Tables | 334 |
| Report coverage | Market Trends, Revenue Estimation and Forecast, Segmentation Analysis, Regional and Country Breakdown, Porter’s 5 Forces Analysis, Company Profiling, Companies Strategic Developments, SWOT Analysis, Winning Imperatives |
| Segments covered | Animal Type, Scope, End User, Region |
| Country scope |
|
| Companies Included | Zoetis, Inc., Virbac, Merck KGaA, Elanco Animal Health, Inc., Boehringer Ingelheim International GmbH, Ceva Sante Animale, Phibro Animal Health Corporation, and Indian Immunologicals Ltd. |
By Animal Type
By Scope
By End User
By Geography
This Market size is expected to reach $4.33 billion by 2032.
Rising Incidence Of Zoonotic And Chronic Animal Diseases are driving the Market in coming years, however, High Cost Of Monoclonal Antibody Development And Treatment restraints the growth of the Market.
Zoetis, Inc., Virbac, Merck KGaA, Elanco Animal Health, Inc., Boehringer Ingelheim International GmbH, Ceva Sante Animale, Phibro Animal Health Corporation, and Indian Immunologicals Ltd.
The expected CAGR of this Market is 18.5% from 2023 to 2032.
The Dermatology segment led the maximum revenue in the Market by Scope in 2024, thereby, achieving a market value of $2.1 billion by 2032.
The North America region dominated the Market by Region in 2024, and would continue to be a dominant market till 2032; thereby, achieving a market value of $1.8 billion by 2032.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

 Drivers
Drivers
 Restraints
Restraints
 Opportunities
Opportunities
 Challenges
Challenges
