The Latin America, Middle East and Africa Medical Device Testing, Inspection And Certification Outsourcing Market would witness market growth of 10.0% CAGR during the forecast period (2025-2032).
The Brazil market dominated the LAMEA Medical Device Testing, Inspection And Certification Outsourcing Market by Country in 2024, and would continue to be a dominant market till 2032; thereby, achieving a market value of $150.5 million by 2032. Argentina market is showcasing a CAGR of 11.3% during (2025 - 2032). Additionally, The South Africa market would register a CAGR of 8.8% during (2025 - 2032).
The market for medical device testing, inspection, and certification (TIC) outsourcing in Latin America, the Middle East, and Africa (LAMEA) has grown to be an essential part of the region's larger healthcare system. Inconsistent regulatory frameworks, restricted access to cutting-edge testing infrastructure, and a lack of harmonization with international standards have historically been problems for the LAMEA region. However, the region's efforts to conform to international regulatory standards have accelerated due to mounting pressure from global trade, growing healthcare demands, and the spread of cutting-edge medical technologies.
Free Valuable Insights: The Worldwide Medical Device Testing, Inspection And Certification Outsourcing Market is Projected to reach USD 5.48 Billion by 2032, at a CAGR of 8.3%
The LAMEA region—comprising Latin America, the Middle East, and Africa—has emerged as a dynamic frontier in the global healthcare landscape, witnessing transformative growth in medical infrastructure, technological adoption, and regulatory evolution. In this context, the Medical Device Testing, Inspection, and Certification (TIC) Outsourcing Market has gained increasing prominence. As manufacturers grapple with complex compliance demands, evolving patient safety standards, and the need to optimize costs, TIC services have become essential in ensuring that medical devices are safe, effective, and market-ready.
The LAMEA region has been progressively aligning its medical device regulations with international standards to ensure product safety and efficacy. This harmonization facilitates easier market entry for manufacturers and ensures that devices meet globally recognized benchmarks.
In Latin America, countries like Brazil and Argentina have strengthened their regulatory frameworks, with agencies such as ANVISA implementing rigorous evaluation processes. These measures aim to enhance the quality of medical devices and protect public health.
The integration of advanced technologies into medical devices has transformed the healthcare landscape, necessitating specialized testing and certification processes. Innovations such as artificial intelligence (AI), the Internet of Things (IoT), and digital health solutions have introduced new complexities in device functionality and data management.
In the LAMEA region, the adoption of these technologies is on the rise, driven by the need to improve patient outcomes and healthcare efficiency. For instance, wearable devices that monitor vital signs in real-time are becoming increasingly popular, enabling remote patient monitoring and early detection of health issues. Similarly, AI-powered diagnostic tools are enhancing the accuracy and speed of disease detection.
Global TIC conglomerates such as TÜV SÜD, SGS, and Intertek have firmly planted their roots in the LAMEA region, targeting countries with evolving but increasingly stringent regulatory systems such as Brazil, the United Arab Emirates, and South Africa. These companies leverage their global reputations, extensive technical capabilities, and familiarity with international regulatory frameworks to serve medical device manufacturers operating in or exporting to these regions.
For example, TÜV SÜD has extended its ISO 13485 certification services into Brazil and South Africa, offering a full suite of regulatory compliance support for medical device producers. Their global accreditation and ability to map country-specific standards—such as ANVISA’s requirements in Brazil—have positioned them as critical enablers of cross-border product deployment.
While global players dominate large-scale and multinational projects, regional and local TIC firms in the LAMEA region have carved out strong niches by leveraging proximity, cultural fluency, and deep regulatory know-how. These firms often fill in gaps left by multinational providers by addressing nuances in local legislation, language barriers, and logistical constraints, especially for small-to-mid-sized device manufacturers.
In Brazil, for instance, the country’s national metrology institute INMETRO certifies local testing labs that comply with ANVISA’s medical device regulations. Local firms like Certi and IQA work closely with domestic producers, enabling quicker turnaround times and reduced costs for conformity assessments. Their fluency in Brazil’s healthcare and technical standards environment makes them indispensable for early-stage device testing and national market access.
Based on Service, the market is segmented into Testing, Inspection, and Certification.
The Testing segment accounted for the largest share of the LAMEA medical device TIC outsourcing market in 2024. Testing involves verifying the safety, efficacy, electrical and mechanical performance, biocompatibility, and sterility of devices. With many countries in the LAMEA region relying on imported devices or producing goods for export, alignment with international standards such as ISO 13485, IEC 60601, and ISO 10993 is critical—making third-party testing essential.
For example, SGS operates specialized medical device testing labs in Egypt and South Africa, offering services such as biocompatibility assessment and electromagnetic compatibility (EMC) testing tailored for African OEMs targeting CE marking. In 2023, SGS collaborated with a major South African contract manufacturer to accelerate testing for surgical kits destined for EU markets.
The Inspection segment held the second-largest share in the LAMEA TIC market. Inspection services are vital for quality assurance, regulatory audits, supply chain verification, and batch-wise release of medical devices. These services are particularly important in LAMEA’s fragmented manufacturing base, where many small to medium enterprises (SMEs) lack in-house capabilities for process validation or equipment inspection.
Bureau Veritas has been a frontrunner in providing inspection services for manufacturing sites in Tunisia and Morocco. The company offers pre-shipment inspections for surgical instruments and personal protective equipment (PPE), which are critical for exports to Europe. In 2023, Bureau Veritas conducted more than 2,000 batch release inspections for disposable syringes exported from Algeria to EU member states.
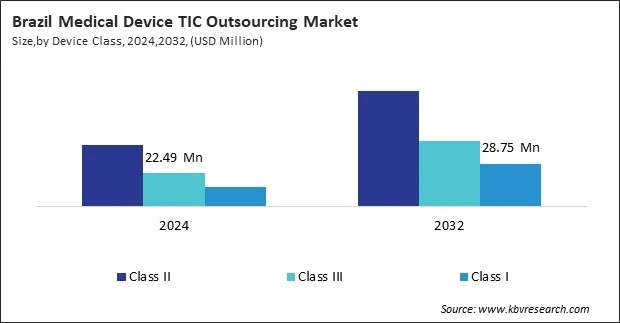
Based on Device Class, the market is segmented into Class II, Class III, and Class I.
The Class II segment accounted for the largest share of the LAMEA TIC outsourcing market in 2024. These devices represent moderate risk and typically require more rigorous evaluation than Class I, but less than the highest-risk Class III devices. Common examples include infusion pumps, surgical drapes, powered wheelchairs, and diagnostic imaging systems.
In Brazil, the Agência Nacional de Vigilância Sanitária (ANVISA) mandates detailed testing and certification for Class II devices, often relying on third-party testing labs accredited under INMETRO schemes. SGS operates one of the largest compliance laboratories in Brazil, performing EMC and electrical safety testing for diagnostic imaging equipment.
Class III medical devices, which carry the highest risk, ranked second in terms of TIC outsourcing demand in the LAMEA region. These include implantable pacemakers, artificial heart valves, and neurostimulators. Due to their complexity and risk profile, Class III devices undergo exhaustive clinical and laboratory evaluations.
In 2024, TÜV SÜD collaborated with a cardiovascular implant startup in the UAE to conduct risk-based performance and biocompatibility testing for their artificial heart valve. The partnership involved both local bench testing and coordination with TÜV SÜD’s global network for in-depth toxicology and accelerated aging studies.
Based on End Use, the market is segmented into Medical Device Companies, Pharmaceutical and Biotech Companies, and Other End Use.
The medical device companies segment is the dominant contributor to the LAMEA TIC outsourcing market. These companies rely heavily on third-party testing, inspection, and certification services to comply with local and international regulatory requirements. LAMEA countries are increasingly aligning with global quality and safety standards such as ISO 13485, IEC 60601, and US FDA guidelines, prompting OEMs to seek reliable TIC partners.
For instance, SGS operates ISO 17025-accredited facilities in Brazil and provides electromagnetic compatibility (EMC) and biocompatibility testing for devices intended for both domestic use and export to Europe and North America. In 2023, TÜV Rheinland partnered with a Middle Eastern startup producing portable dialysis machines to provide safety and performance certification under the Gulf Cooperation Council (GCC) regulatory framework.
While not the primary users, pharmaceutical and biotech companies represent a significant and growing share of the LAMEA TIC outsourcing landscape. Their need for TIC services stems from the increasing integration of combination products, such as drug-device systems, autoinjectors, and inhalers, which require compliance with both drug and device regulations.
For example, Bureau Veritas has provided end-to-end compliance testing for a Saudi Arabian pharma company developing insulin pens, including biocompatibility, mechanical safety, and sterilization validation. Similarly, UL Solutions supported a Brazilian biologics manufacturer with ISO 11607 packaging validation for a syringe-based monoclonal antibody delivery system.
By Service
By Device Class
By End Use
By Country
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

