The Global Implantable Loop Recorders Market size is expected to reach $2.1 billion by 2026, rising at a market growth of 11.9% CAGR during the forecast period. The implantable loop recorder (ILR) is a portable cardiac recording system capable of continuously saving ECG data in the case of bradyarrhythmia or tachyarrhythmia. This is inserted under the skin where the pocket on the left side of the breastbone is shaped. ILR is often seen in people with conditions such as syncope, repeated palpitations, skipped beats, light-headedness, or dizziness.
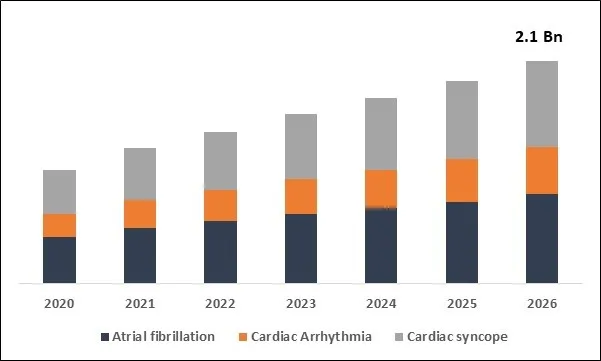
Global Implantable Loop Recorders Market Size
Syncope is a sudden loss of control caused by systemic arterial hypotension related to excessive vasodilatation or bradycardia or both. Unexplained Syncope applies to the form of syncope where no specific cardiologic or neurological explanation can be made. Implantable loop recorders (ILRs) may help try to discern between various types of temporary lack of consciousness.
The ILR has a safe and effective way to monitor patients with recurrent unexplained syncope. ILR is a subcutaneous, single-lead, electrocardiographic (ECG) monitoring system used for the treatment of patients with chronic abnormal palpitation or syncope events, coupled with long-term surveillance of patients at risk of or with atrial fibrillation, systemic heart failure, bundle branch obstruction, persistent neural induced syncope, etc. This consists of an insertable unit, which comes with a battery and two surface electrodes.
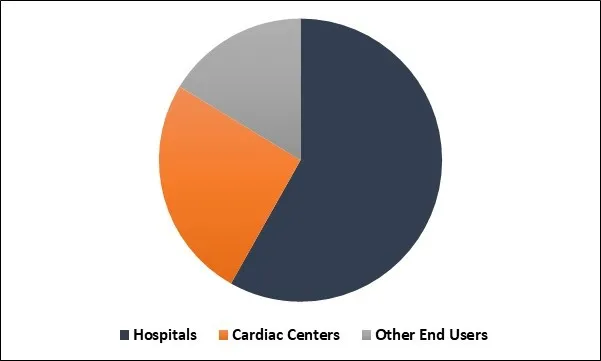
Implantable Loop Recorders Market Share
Technological advances are anticipated to play a vital role in the introduction of new-age implantable loop recorders. Stakeholders competing in the existing business environment implantable loop recorders are anticipated to develop stronger and 'smarter' implantable loop recorders during the prediction timeframe to increase their consumer presence. Manufacturers in the market for implantable loop recorders are introducing new products that can be attached to mobile applications through Bluetooth and Wi-Fi.
Free Valuable Insights: Global Implantable Loop Recorders Market to reach a market size of $2.1 billion by 2026
Based on Application, the market is segmented into Atrial fibrillation, Cardiac Arrhythmia and Cardiac syncope. Based on End User, the market is segmented into Hospitals, Cardiac Centers and Other End Users. Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Medtronic PLC, Abbott Laboratories, Boston Scientific Corporation, BIOTRONIK SE & Co. KG, Angel Medical Systems, Inc., and Vectorious Medical Technologies Ltd.
Market Segmentation:
By Application
By End-User
By Geography
Companies Profiled
The implantable loop recorders market is projected to reach USD 2.1 billion by 2026.
The major factors that are anticipated to drive the implantable loop recorders industry include demand for remote patient monitoring amid the coronavirus (COVID-19) outbreak.
Medtronic PLC, Abbott Laboratories, Boston Scientific Corporation, BIOTRONIK SE & Co. KG, Angel Medical Systems, Inc., and Vectorious Medical Technologies Ltd.
The hospital segment recorded a dominant market share. The dominance of the segment may be attributed to an improvement in the number of minimally invasive surgeries in such hospitals.
Our team of dedicated experts can provide you with attractive expansion opportunities for your business.

