The Medical Device Testing, Inspection And Certification Outsourcing Market is Predict to reach USD 5.48 Billion by 2032, at a CAGR of 8.3%
Special Offering :
Industry Insights | Market Trends | Highest number of Tables | 24/7 Analyst Support
Medical Device Testing, Inspection And Certification Outsourcing Market Growth, Trends and Report Highlights
According to a new report, published by KBV research, The Global Medical Device Testing, Inspection And Certification Outsourcing Market size is expected to reach $5.48 billion by 2032, rising at a market growth of 8.3% CAGR during the forecast period.
The global medical device testing, inspection, and certification (TIC) outsourcing market has evolved significantly over the past few decades, driven by the rapid advancement of medical technologies, heightened regulatory scrutiny, and the globalization of healthcare product distribution. Initially, many medical device manufacturers managed quality assurance and regulatory compliance processes internally.
The Inspection segment is experiencing a CAGR of 9% during (2025 - 2032). Inspection services include in-process inspections, final inspections, and factory audits, all designed to ensure adherence to regulatory requirements and quality standards. Medical device manufacturers utilize these services to verify that raw materials, production processes, and finished goods meet expected specifications. Additionally, outsourcing inspection tasks helps companies maintain consistency, reduce operational risks, and respond quickly to quality concerns across global supply chains.
The Class II segment is leading the Global Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class in 2024; thereby, achieving a market value of $2.8 billion by 2032. These include infusion pumps, surgical drapes, and powered wheelchairs, which require rigorous performance and safety evaluations. While not as high-risk as Class III devices, Class II products often demand premarket notifications and special controls, increasing the need for professional testing and certification services. Manufacturers increasingly outsource these services to ensure compliance with regional and global regulatory frameworks and to streamline product approvals.
The Pharmaceutical and Biotech Companies’ segment would register a CAGR of 9.3% during (2025 - 2032). These firms require medical device testing services to ensure that the mechanical and functional components of their products meet quality and safety standards. As regulatory bodies expand oversight over such hybrid products, pharmaceutical and biotech companies seek external partners for device compatibility testing, materials evaluation, and certification support. This collaboration ensures successful market entry and mitigates compliance risks throughout the product lifecycle.
Full Report: https://www.kbvresearch.com/medical-device-testing-inspection-and-certification-outsourcing-market/
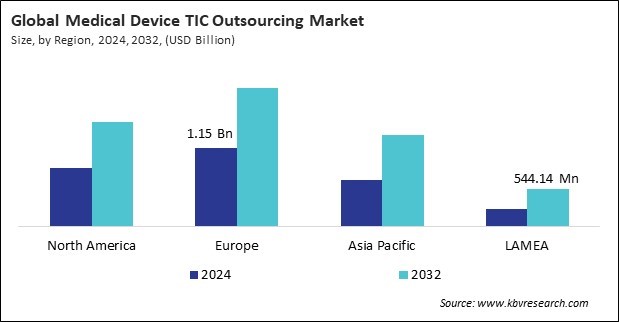
The Europe region dominated the Global Medical Device Testing, Inspection And Certification Outsourcing Market by Region in 2024, and would continue to be a dominant market till 2032; thereby, achieving a market value of $2.04 billion by 2032. The North America region is experiencing a CAGR of 7.9% during (2025 - 2032). Additionally, The Asia Pacific region would exhibit a CAGR of 9.2% during (2025 - 2032).
List of Key Companies Profiled
- SGS S.A.
- Intertek Group PLC
- Eurofins Scientific SE
- TUV SUD
- ALS Limited
- Bureau Veritas S.A.
- Element Materials Technology (Temasek Holdings)
- DNV AS
- Pace Analytical Services, LLC
- Nelson Laboratories, LLC (Sotera Health Company)
Medical Device Testing, Inspection And Certification Outsourcing Market Report Segmentation
By Service
- Testing
- Inspection
- Certification
By Device Class
- Class II
- Class III
- Class I
By End Use
- Medical Device Companies
- Pharmaceutical and Biotech Companies
- Other End Use
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Related Reports: