The Dietary Supplement Testing Market is Predict to reach USD 4.05 Billion by 2032, at a CAGR of 8.5%
Special Offering :
Industry Insights | Market Trends | Highest number of Tables | 24/7 Analyst Support
Dietary Supplement Testing Market Growth, Trends and Report Highlights
According to a new report, published by KBV research, The Global Dietary Supplement Testing Market size is expected to reach $4.05 billion by 2032, rising at a market growth of 8.5% CAGR during the forecast period.
The global dietary supplement testing market has developed as a natural extension of food safety and pharmaceutical quality control, evolving over the past century into a specialized and indispensable field. Dietary supplements, unlike conventional foods, are consumed in concentrated forms—such as capsules, powders, or liquids—and are often intended to address nutrient deficiencies, enhance performance, or promote general health.
The Traditional Testing segment is poised to grow at a CAGR of 7.8 % during the forecast period. These methods are highly accurate and cover critical areas such as contaminants detection, potency measurement, identity verification, and stability testing. Traditional testing provides comprehensive insights that are essential for meeting stringent regulations set by global authorities, including the FDA, EFSA, and other international bodies. Its reliability makes it the preferred choice for manufacturers and regulators, particularly in a market where consumer trust and transparency are fundamental to growth.
The Ingredient-Level Testing segment captured the maximum revenue in the Global Dietary Supplement Testing Market by Ingredient Type in 2024, thereby, achieving a market value of $2.1 billion by 2032. With supplements heavily reliant on botanicals, herbal extracts, vitamins, and minerals sourced from across diverse geographies, ingredient-level testing is crucial in identifying contaminants, confirming species identity, and validating active compound concentrations. This process reduces the risks of adulteration, mislabeling, and inconsistent quality, while also supporting regulatory compliance with international standards.
The Independent Third-Party Testing Laboratories segment is experiencing a CAGR of 8.1 % during the forecast period. These laboratories provide essential services such as contaminant detection, potency validation, authenticity checks, and label claim verification, which are critical for maintaining consumer confidence and ensuring compliance with international standards. Their neutrality makes them a preferred choice for manufacturers, distributors, and regulators alike, particularly in a global market where transparency and traceability are paramount.
The Nutraceutical Companies segment led the maximum revenue in the Global Dietary Supplement Testing Market by End User in 2024, thereby, achieving a market value of $1.6 billion by 2032. The nutraceutical companies segment represents the largest share of the global dietary supplement testing market, as these companies are directly responsible for ensuring that their products meet safety, potency, and quality standards. With rising consumer demand for clean-label, science-backed supplements, testing has become an indispensable part of product development and manufacturing.
The Contaminants (heavy metals, pesticides, solvents) segment is growing at a CAGR of 6.6 % during the forecast period. With the supplement industry sourcing raw materials from diverse regions, including areas where agricultural and processing practices vary widely, contaminants testing provides a critical safeguard. It ensures compliance with strict global regulations, reduces the risk of harmful exposure, and supports international trade by meeting the requirements of agencies such as the U.S. FDA, the European Food Safety Authority (EFSA), and others.
Full Report: https://www.kbvresearch.com/dietary-supplement-testing-market/
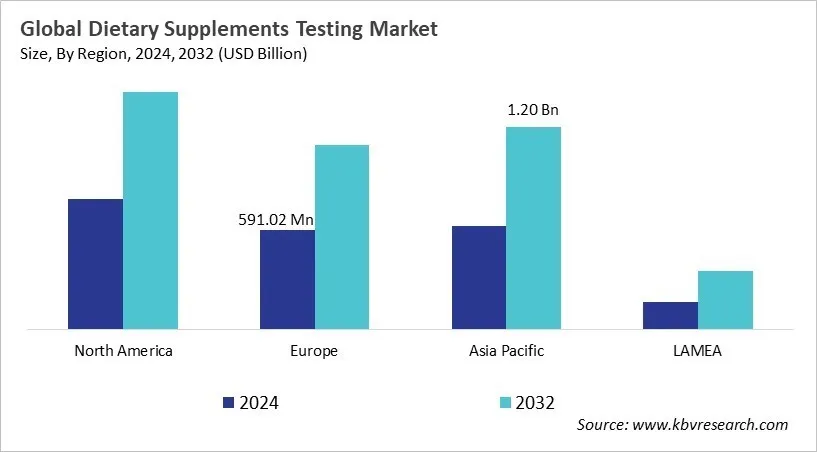
The North America region dominated the Global Dietary Supplement Testing Market by Region in 2024, and would continue to be a dominant market till 2032; thereby, achieving a market value of $1.4 billion by 2032. The Asia Pacific region is anticipated to grow at a CAGR of 9% during (2025 - 2032). Additionally, The Europe region would witness a CAGR of 8.2% during (2025 - 2032).
List of Key Companies Profiled
- Eurofins Scientific SE
- Tentamus Group GmbH
- Intertek Group PLC
- Alkemist Labs
- SGS S.A.
- AGROLAB GmbH
- Anresco, Inc.
- FoodChain ID Group, Inc.
- BeaconPointLabs, LLC
- Certified Laboratories, LLC
Dietary Supplement Testing Market Report Segmentation
By Technology
- Traditional Testing
- Rapid Testing
By Ingredient Type
- Ingredient-Level Testing
- Finished Product Testing
By Service Provider
- Contract Research Organizations (CROs)
- Independent Third-Party Testing Laboratories
- Other Service Provider
By End User
- Nutraceutical Companies
- Contract Manufacturers
- Distributors / Label Claim Verificationers (Online & Offline)
- Regulatory Authorities
- Other End User
By Test Type
- Contaminants (heavy metals, pesticides, solvents)
- Microbiological
- Potency
- Identity / Authentication
- Adulteration
- Label Claim Verification
- Stability & Shelf Life
- Allergen & GMO Testing
- Other Test Types
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Related Reports: