North America Bioprocess Validation Market Size, Share & Industry Analysis Report By Mode (In house and Outsourced), By Stage (Continued Process Verification, Process Qualification, and Process Design), By Testing Type, By Country and Growth Forecast, 2025 - 2032
Published Date : 03-Oct-2025 |
Pages: 154 |
Report Format: PDF + Excel |
COVID-19 Impact on the North America Bioprocess Validation Market
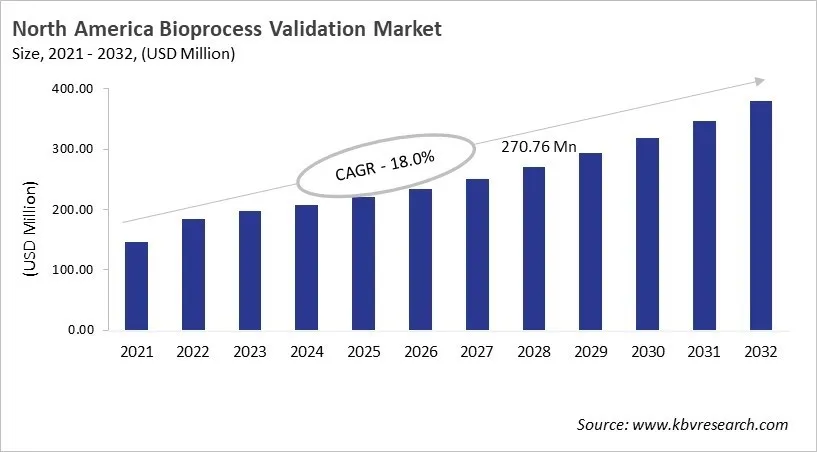
The North America Bioprocess Validation Market would witness market growth of 8.1% CAGR during the forecast period (2025-2032).
The US market dominated the North America Bioprocess Validation Market by Country in 2024, and would continue to be a dominant market till 2032; thereby, achieving a market value of USD 264.2 million by 2032. The Canada market is experiencing a CAGR of 9.9% during (2025 - 2032). Additionally, The Mexico market would exhibit a CAGR of 9.6% during (2025 - 2032). The US and Canada led the North America Bioprocess Validation Market by Country with a market share of 73.4% and 15% in 2024.
The North American bioprocess validation market has changed from basic end-point checks to a full discipline based on the lifecycle that supports modern biopharmaceutical manufacturing. Early validation was mostly about making sure that equipment was safe to use. But as biologics, advanced vaccines, and recombinant therapies became more popular, regulatory agencies started to require ongoing monitoring and compliance at the design stage. New validation methods were needed to check the integrity and compatibility of new technologies like single-use systems, disposable bioreactor bags, and continuous manufacturing. Digital platforms and process analytical technologies now make it possible to collect data in real time. This turns validation into an ongoing process that makes sure products are reliable and can be released more quickly. Outsourcing has grown a lot, and specialized service providers help biopharma companies stay compliant with regulations while focusing on their core research and development.
Three big changes in the market right now are the use of real-time, data-driven validation, the need for extensive validation of single-use and modular systems, and the growing reliance on specialized outsourcing. Companies spend a lot of money on digital tools, automated testing, and predictive analytics to speed up processes and make better decisions. Top companies package equipment with validation services, move into biotech hubs, and make plans for new therapies like cell and gene therapies. Global equipment suppliers with integrated solutions, independent validation firms that offer unbiased expertise, and regional players that offer flexible, affordable services all shape the competition. A moderately consolidated but very dynamic market landscape is necessary for success. This is because regulatory credibility, strong documentation, and new ideas in automation and analytics are all important.
Testing Type Outlook
Based on Testing Type, the market is segmented into Bioprocess Residuals Testing, Extractables & Leachables Testing, Viral Clearance Testing, Wireless and IoT Penetration Testing, and Other Testing Type. Among various US Bioprocess Validation Market by Testing Type; The Bioprocess Residuals Testing market achieved a market size of USD $42.6 Million in 2024 and is expected to grow at a CAGR of 6.4 % during the forecast period. The Viral Clearance Testing market is predicted to experience a CAGR of 8% throughout the forecast period from (2025 - 2032).
Mode Outlook
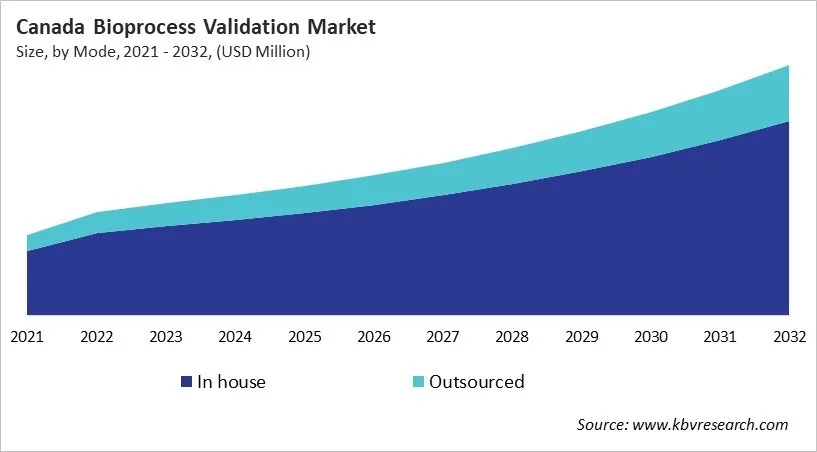
Based on Mode, the market is segmented into In house and Outsourced. The In house market segment dominated the Canada Bioprocess Validation Market by Mode is expected to grow at a CAGR of 9.6 % during the forecast period thereby continuing its dominance until 2032. Also, The Outsourced market is anticipated to grow as a CAGR of 11.2 % during the forecast period during (2025 - 2032).
Free Valuable Insights: The Bioprocess Validation Market is Predict to reach USD 994 Million by 2032, at a CAGR of 8.8%
Country Outlook
The US is the leader in the bioprocess validation market because it has a strong biopharmaceutical industry, strict FDA rules, and a lot of money goes into research and development. The need for accurate, fully validated manufacturing processes is growing because more people want biologics, biosimilars, and advanced therapies like cell and gene treatments. Market trends show that more and more people are using single-use technologies, modular systems, automation, and real-time data analytics to make systems more scalable, lower the risk of contamination, and allow for predictive maintenance. There is a lot of competition, with big companies like Thermo Fisher, Danaher, Merck KGaA, Sartorius, and Lonza, as well as many CDMOs that offer specialized validation services. To stay ahead of the competition, top companies are adding more services, using more advanced analytical tools, and working with schools and businesses. Market growth will continue because of ongoing regulatory pressure and the move toward more efficient and flexible manufacturing.
List of Key Companies Profiled
- Merck KGaA
- Thermo Fisher Scientific, Inc.
- SGS S.A.
- Eurofins Scientific SE
- Sartorius AG
- Charles River Laboratories International, Inc.
- Lonza Group Ltd.
- WuXi AppTec Co., Ltd.
- Danaher Corporation
- Cobetter Filtration equipment Co., Ltd.
North America Bioprocess Validation Market Report Segmentation
By Mode
- In house
- Outsourced
By Stage
- Continued Process Verification
- Process Qualification
- Process Design
By Testing Type
- Bioprocess Residuals Testing
- Extractables & Leachables Testing
- Viral Clearance Testing
- Wireless and IoT Penetration Testing
- Other Testing Type
By Country
- US
- Canada
- Mexico
- Rest of North America
1.1 Market Definition
1.2 Objectives
1.3 Market Scope
1.4 Segmentation
1.4.1 North America Bioprocess Validation Market, by Mode
1.4.2 North America Bioprocess Validation Market, by Stage
1.4.3 North America Bioprocess Validation Market, by Testing Type
1.4.4 North America Bioprocess Validation Market, by Country
1.5 Methodology for the research
Chapter 2. Market at a Glance
2.1 Key Highlights
Chapter 3. Market Overview
3.1 Introduction
3.1.1 Overview
3.1.1.1 Market Composition and Scenario
3.2 Key Factors Impacting the Market
3.2.1 Market Drivers
3.2.2 Market Restraints
3.2.3 Market Opportunities
3.2.4 Market Challenges
Chapter 4. Market Trends – North America Bioprocess Validation Market
Chapter 5. State of Competition – North America Bioprocess Validation Market
Chapter 6. Value Chain Analysis of Bioprocess Validation Market
Chapter 7. Product Life Cycle – Bioprocess Validation Market
Chapter 8. Market Consolidation – Bioprocess Validation Market
Chapter 9. Key Customer Criteria – Bioprocess Validation Market
Chapter 10. Competition Analysis – Global
10.1 Market Share Analysis, 2024
10.2 Recent Strategies Deployed in Bioprocess Validation Market
10.3 Porter Five Forces Analysis
Chapter 11. North America Bioprocess Validation Market by Mode
11.1 North America In house Market by Region
11.2 North America Outsourced Market by Region
Chapter 12. North America Bioprocess Validation Market by Stage
12.1 North America Continued Process Verification Market by Country
12.2 North America Process Qualification Market by Country
12.3 North America Process Design Market by Country
Chapter 13. North America Bioprocess Validation Market by Testing Type
13.1 North America Bioprocess Residuals Testing Market by Country
13.2 North America Extractables & Leachables Testing Market by Country
13.3 North America Viral Clearance Testing Market by Country
13.4 North America Wireless and IoT Penetration Testing Market by Country
13.5 North America Other Testing Type Market by Country
Chapter 14. North America Bioprocess Validation Market by Country
14.1 US Bioprocess Validation Market
14.1.1 US Bioprocess Validation Market by Mode
14.1.2 US Bioprocess Validation Market by Stage
14.1.3 US Bioprocess Validation Market by Testing Type
14.2 Canada Bioprocess Validation Market
14.2.1 Canada Bioprocess Validation Market by Mode
14.2.2 Canada Bioprocess Validation Market by Stage
14.2.3 Canada Bioprocess Validation Market by Testing Type
14.3 Mexico Bioprocess Validation Market
14.3.1 Mexico Bioprocess Validation Market by Mode
14.3.2 Mexico Bioprocess Validation Market by Stage
14.3.3 Mexico Bioprocess Validation Market by Testing Type
14.4 Rest of North America Bioprocess Validation Market
14.4.1 Rest of North America Bioprocess Validation Market by Mode
14.4.2 Rest of North America Bioprocess Validation Market by Stage
14.4.3 Rest of North America Bioprocess Validation Market by Testing Type
Chapter 15. Company Profiles
15.1 Merck KGaA
15.1.1 Company Overview
15.1.2 Financial Analysis
15.1.3 Segmental and Regional Analysis
15.1.4 Research & Development Expenses
15.1.5 Recent strategies and developments:
15.1.5.1 Partnerships, Collaborations, and Agreements:
15.1.6 SWOT Analysis
15.2 Thermo Fisher Scientific, Inc.
15.2.1 Company Overview
15.2.2 Financial Analysis
15.2.3 Segmental and Regional Analysis
15.2.4 Research & Development Expenses
15.2.5 Recent strategies and developments:
15.2.5.1 Product Launches and Product Expansions:
15.2.6 SWOT Analysis
15.3 SGS S.A.
15.3.1 Company Overview
15.3.2 Financial Analysis
15.3.3 Segmental and Regional Analysis
15.3.4 SWOT Analysis
15.4 Eurofins Scientific SE
15.4.1 Company Overview
15.4.2 Financial Analysis
15.4.3 Regional Analysis
15.4.4 SWOT Analysis
15.5 Sartorius AG
15.5.1 Company Overview
15.5.2 Financial Analysis
15.5.3 Segmental and Regional Analysis
15.5.4 Research & Development Expenses
15.5.5 Recent strategies and developments:
15.5.5.1 Partnerships, Collaborations, and Agreements:
15.5.5.2 Geographical Expansions:
15.5.6 SWOT Analysis
15.6 Charles River Laboratories International, Inc.
15.6.1 Company Overview
15.6.2 Financial Analysis
15.6.3 Segmental and Regional Analysis
15.6.4 SWOT Analysis
15.7 Lonza Group Ltd.
15.7.1 Company Overview
15.7.2 inancial Analysis
15.7.3 Segmental and Regional Analysis
15.7.4 Research & Development Expenses
15.7.5 Recent strategies and developments:
15.7.5.1 Product Launches and Product Expansions:
15.7.6 SWOT Analysis
15.8 WuXi AppTec Co., Ltd.
15.8.1 Company Overview
15.8.2 Financial Analysis
15.8.3 Segmental Analysis
15.8.4 Research & Development Expenses
15.9 Danaher Corporation
15.9.1 Company Overview
15.9.2 Financial Analysis
15.9.3 Segmental and Regional Analysis
15.9.4 Research & Development Expense
15.9.5 SWOT Analysis
15.1 Cobetter Filtration equipment Co., Ltd.
15.10.1 Company Overview
TABLE 2 North America Bioprocess Validation Market, 2025 - 2032, USD Million
TABLE 3 Key Customer Criteria – Bioprocess Validation Market
TABLE 4 North America Bioprocess Validation Market by Mode, 2021 - 2024, USD Million
TABLE 5 North America Bioprocess Validation Market by Mode, 2025 - 2032, USD Million
TABLE 6 North America In house Market by Region, 2021 - 2024, USD Million
TABLE 7 North America In house Market by Region, 2025 - 2032, USD Million
TABLE 8 North America Outsourced Market by Region, 2021 - 2024, USD Million
TABLE 9 North America Outsourced Market by Region, 2025 - 2032, USD Million
TABLE 10 North America Bioprocess Validation Market by Stage, 2021 - 2024, USD Million
TABLE 11 North America Bioprocess Validation Market by Stage, 2025 - 2032, USD Million
TABLE 12 North America Continued Process Verification Market by Country, 2021 - 2024, USD Million
TABLE 13 North America Continued Process Verification Market by Country, 2025 - 2032, USD Million
TABLE 14 North America Process Qualification Market by Country, 2021 - 2024, USD Million
TABLE 15 North America Process Qualification Market by Country, 2025 - 2032, USD Million
TABLE 16 North America Process Design Market by Country, 2021 - 2024, USD Million
TABLE 17 North America Process Design Market by Country, 2025 - 2032, USD Million
TABLE 18 North America Bioprocess Validation Market by Testing Type, 2021 - 2024, USD Million
TABLE 19 North America Bioprocess Validation Market by Testing Type, 2025 - 2032, USD Million
TABLE 20 North America Bioprocess Residuals Testing Market by Country, 2021 - 2024, USD Million
TABLE 21 North America Bioprocess Residuals Testing Market by Country, 2025 - 2032, USD Million
TABLE 22 North America Extractables & Leachables Testing Market by Country, 2021 - 2024, USD Million
TABLE 23 North America Extractables & Leachables Testing Market by Country, 2025 - 2032, USD Million
TABLE 24 North America Viral Clearance Testing Market by Country, 2021 - 2024, USD Million
TABLE 25 North America Viral Clearance Testing Market by Country, 2025 - 2032, USD Million
TABLE 26 North America Wireless and IoT Penetration Testing Market by Country, 2021 - 2024, USD Million
TABLE 27 North America Wireless and IoT Penetration Testing Market by Country, 2025 - 2032, USD Million
TABLE 28 North America Other Testing Type Market by Country, 2021 - 2024, USD Million
TABLE 29 North America Other Testing Type Market by Country, 2025 - 2032, USD Million
TABLE 30 North America Bioprocess Validation Market by Country, 2021 - 2024, USD Million
TABLE 31 North America Bioprocess Validation Market by Country, 2025 - 2032, USD Million
TABLE 32 US Bioprocess Validation Market, 2021 - 2024, USD Million
TABLE 33 US Bioprocess Validation Market, 2025 - 2032, USD Million
TABLE 34 US Bioprocess Validation Market by Mode, 2021 - 2024, USD Million
TABLE 35 US Bioprocess Validation Market by Mode, 2025 - 2032, USD Million
TABLE 36 US Bioprocess Validation Market by Stage, 2021 - 2024, USD Million
TABLE 37 US Bioprocess Validation Market by Stage, 2025 - 2032, USD Million
TABLE 38 US Bioprocess Validation Market by Testing Type, 2021 - 2024, USD Million
TABLE 39 US Bioprocess Validation Market by Testing Type, 2025 - 2032, USD Million
TABLE 40 Canada Bioprocess Validation Market, 2021 - 2024, USD Million
TABLE 41 Canada Bioprocess Validation Market, 2025 - 2032, USD Million
TABLE 42 Canada Bioprocess Validation Market by Mode, 2021 - 2024, USD Million
TABLE 43 Canada Bioprocess Validation Market by Mode, 2025 - 2032, USD Million
TABLE 44 Canada Bioprocess Validation Market by Stage, 2021 - 2024, USD Million
TABLE 45 Canada Bioprocess Validation Market by Stage, 2025 - 2032, USD Million
TABLE 46 Canada Bioprocess Validation Market by Testing Type, 2021 - 2024, USD Million
TABLE 47 Canada Bioprocess Validation Market by Testing Type, 2025 - 2032, USD Million
TABLE 48 Mexico Bioprocess Validation Market, 2021 - 2024, USD Million
TABLE 49 Mexico Bioprocess Validation Market, 2025 - 2032, USD Million
TABLE 50 Mexico Bioprocess Validation Market by Mode, 2021 - 2024, USD Million
TABLE 51 Mexico Bioprocess Validation Market by Mode, 2025 - 2032, USD Million
TABLE 52 Mexico Bioprocess Validation Market by Stage, 2021 - 2024, USD Million
TABLE 53 Mexico Bioprocess Validation Market by Stage, 2025 - 2032, USD Million
TABLE 54 Mexico Bioprocess Validation Market by Testing Type, 2021 - 2024, USD Million
TABLE 55 Mexico Bioprocess Validation Market by Testing Type, 2025 - 2032, USD Million
TABLE 56 Rest of North America Bioprocess Validation Market, 2021 - 2024, USD Million
TABLE 57 Rest of North America Bioprocess Validation Market, 2025 - 2032, USD Million
TABLE 58 Rest of North America Bioprocess Validation Market by Mode, 2021 - 2024, USD Million
TABLE 59 Rest of North America Bioprocess Validation Market by Mode, 2025 - 2032, USD Million
TABLE 60 Rest of North America Bioprocess Validation Market by Stage, 2021 - 2024, USD Million
TABLE 61 Rest of North America Bioprocess Validation Market by Stage, 2025 - 2032, USD Million
TABLE 62 Rest of North America Bioprocess Validation Market by Testing Type, 2021 - 2024, USD Million
TABLE 63 Rest of North America Bioprocess Validation Market by Testing Type, 2025 - 2032, USD Million
TABLE 64 key Information – Merck KGaA
TABLE 65 Key Information – Thermo Fisher Scientific, Inc.
TABLE 66 Key Information – SGS S.A.
TABLE 67 Key Information – Eurofins Scientific SE
TABLE 68 Key Information – Eurofins Scientific SE
TABLE 69 key information – Charles River Laboratories International, Inc.
TABLE 70 Key Information –Lonza Group Ltd.
TABLE 71 Key Information – WuXi AppTec Co., Ltd.
TABLE 72 Key Information – Danaher Corporation
TABLE 73 Key Information – Cobetter filtration equipment co., ltd.
List of Figures
FIG 1 Methodology for the research
FIG 2 North America Bioprocess Validation Market, 2021 - 2032, USD Million
FIG 3 Key Factors Impacting north america Bioprocess Validation Market
FIG 4 Value Chain Analysis of Bioprocess Validation Market
FIG 5 Product Life Cycle – Bioprocess Validation Market
FIG 6 Market Consolidation – Bioprocess Validation Market
FIG 7 Key Customer Criteria – Bioprocess Validation Market
FIG 8 Market Share Analysis, 2024
FIG 9 Porter’s Five Forces Analysis – Bioprocess Validation Market
FIG 10 North America Bioprocess Validation Market share by Mode, 2024
FIG 11 North America Bioprocess Validation Market share by Mode, 2032
FIG 12 North America Bioprocess Validation Market by Mode, 2021 - 2032, USD Million
FIG 13 North America Bioprocess Validation Market share by Stage, 2024
FIG 14 North America Bioprocess Validation Market share by Stage, 2032
FIG 15 North America Bioprocess Validation Market by Stage, 2021 - 2032, USD Million
FIG 16 North America Bioprocess Validation Market share by Testing Type, 2024
FIG 17 North America Bioprocess Validation Market share by Testing Type, 2032
FIG 18 North America Bioprocess Validation Market by Testing Type, 2021 - 2032, USD Million
FIG 19 North America Bioprocess Validation Market share by Country, 2024
FIG 20 North America Bioprocess Validation Market share by Country, 2032
FIG 21 North America Bioprocess Validation Market by Country, 2021 - 2032, USD Million
FIG 22 Swot Analysis: Merck KGaA
FIG 23 Swot Analysis: hermo Fisher Scientific, Inc.
FIG 24 Swot Analysis: SGS S.A.
FIG 25 SWOT Analysis: Eurofins Scientific SE
FIG 26 Recent strategies and developments: Sartorius AG
FIG 27 SWOT Analysis: Sartorius AG
FIG 28 Swot Analysis: Charles River Laboratories International, Inc.
FIG 29 Swot Analysis: Lonza Group Ltd.
FIG 30 SWOT Analysis: Danaher Corporation