Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market Size, Share & Industry Analysis Report By Service (Testing, Inspection, and Certification), By Device Class (Class II, Class III, and Class I), By End Use (Medical Device Companies, Pharmaceutical and Biotech Companies, and Other End Use), By Country and Growth Forecast, 2025 - 2032
Published Date : 28-Jul-2025 |
Pages: 168 |
Report Format: PDF + Excel |
COVID-19 Impact on the Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market
The Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market would witness market growth of 9.2% CAGR during the forecast period (2025-2032).
The China market dominated the Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Country in 2024, and would continue to be a dominant market till 2032; thereby, achieving a market value of $426.8 million by 2032. The Japan market is registering a CAGR of 8.5% during (2025 - 2032). Additionally, The India market would showcase a CAGR of 9.9% during (2025 - 2032).
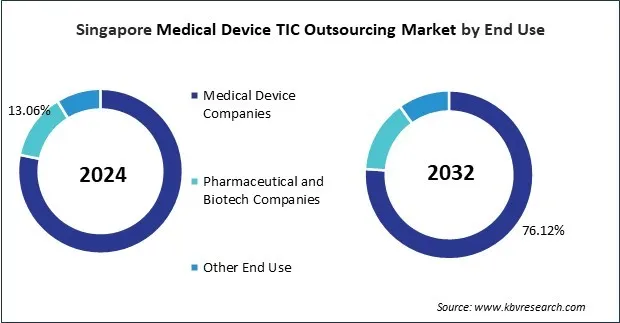
The Asia Pacific medical device TIC outsourcing market has grown along with the region's healthcare infrastructure, the need for better medical devices, and the growing complexity of regulatory frameworks. First, many local and international OEMs handled compliance processes in-house. However, the high costs and different rules in different countries in Asia Pacific quickly made it clear that outsourcing TIC services was a better way to save money and reduce risk. Japan, China, India, and South Korea are now the main places where medical devices are made because they have strong industrial bases and government incentives.
Free Valuable Insights: The Global Medical Device Testing, Inspection And Certification Outsourcing Market is Predict to reach USD 5.48 Billion by 2032, at a CAGR of 8.3%
India’s Push for Local Medical Device Growth
- Government Support Programs: India has launched schemes like the Production Linked Incentive (PLI) and Medical Device Parks to encourage local manufacturing.
- Better Compliance Standards: These efforts also help manufacturers meet higher quality and safety standards for medical devices.
Regional and Global Influence on TIC Services
- More Global Companies, More Testing Needs: With more international companies entering India and Southeast Asia, there’s a growing need for third-party testing and certification services (TIC).
- Easier Regional Trade in ASEAN: Countries in Southeast Asia are working together through the ASEAN Medical Device Directive (AMDD) to make rules more uniform.
- Boost to Outsourced Services: These harmonized rules make it easier for companies to trade and rely on outside experts for regulatory approval and testing.
Market Trends – Asia Pacific
Changes in demographics, rising healthcare costs, changes in regulations, and rapid technological progress are all having a big impact on the healthcare and medical device industries in the Asia Pacific region. At the same time, the need for safe, reliable, and compliant medical devices has skyrocketed, making it necessary to thoroughly test them at every stage of design, development, and deployment. In this situation, Testing, Inspection, and Certification (TIC) services are becoming increasingly important for making sure that medical devices meet both domestic and international rules. As medical devices become more complicated, often with built-in software, IoT integration, and advanced materials, original equipment manufacturers (OEMs) and suppliers are turning to third-party TIC outsourcing partners increasingly for help with compliance and specialized knowledge.
Integration of Artificial Intelligence (AI) and Automation in TIC Processes
The Asia Pacific region is witnessing a transformative shift in the medical device TIC outsourcing market, driven by the integration of Artificial Intelligence (AI) and automation technologies. This trend is reshaping the landscape by enhancing efficiency, accuracy, and compliance in testing, inspection, and certification processes.
AI and automation are being employed to streamline complex TIC procedures, reducing human error and accelerating time-to-market for medical devices. For instance, AI algorithms can analyze vast datasets to identify patterns and anomalies, enabling predictive maintenance and real-time quality control. Automation of repetitive tasks, such as data entry and report generation, frees up human resources for more strategic activities.
Emphasis on Quality Assurance and Regulatory Compliance
Quality assurance and regulatory compliance have become focal points in the Asia Pacific medical device TIC outsourcing market. The increasing complexity of medical devices, coupled with stringent regulatory requirements, has heightened the demand for comprehensive quality assurance services.
Manufacturers are seeking TIC partners that can provide end-to-end quality validation, encompassing design validation, pre-clinical testing, clinical trials, and post-market surveillance. This comprehensive approach ensures that medical devices meet the necessary standards throughout their lifecycle.
State of Competition:
The Asia Pacific medical device industry is experiencing dynamic growth, underpinned by an expanding population, rising healthcare investments, and increasing demand for technologically advanced and regulatory-compliant medical solutions. As these developments unfold, the Testing, Inspection, and Certification (TIC) outsourcing segment has emerged as a critical enabler, ensuring that medical devices meet rigorous safety, quality, and regulatory benchmarks across diverse national markets. The competitive landscape within this domain is evolving rapidly, shaped by the entry of global testing giants, the rise of agile regional players, the integration of TIC functions within contract manufacturing organizations, and the growing influence of digital technologies.
1. Dominance of Global TIC Giants in High-Compliance Markets
Global TIC conglomerates such as TÜV SÜD, SGS, and Intertek have established a strong foothold in the Asia Pacific region, particularly in countries with stringent regulatory environments like Japan, South Korea, and Singapore. Their extensive experience in navigating complex international standards positions them as preferred partners for multinational medical device manufacturers seeking consistent compliance across multiple markets.
For instance, TÜV SÜD operates accredited laboratories in key Asian hubs, offering comprehensive services that align with both local and international regulatory requirements. Their collaboration with local regulatory bodies ensures a seamless certification process for clients. Similarly, SGS has expanded its presence in Asia by establishing state-of-the-art testing facilities that cater to the diverse needs of the medical device industry. These facilities are equipped to handle a wide range of testing protocols, ensuring that devices meet the necessary safety and efficacy standards before entering the market.
Intertek, another global player, has leveraged its vast network to provide end-to-end TIC services, including regulatory consulting, quality assurance, and clinical trial support. Their integrated approach allows for efficient project management and faster time-to-market for medical devices.
2. Emergence of Regional Specialists Catering to Local Market Needs
While global TIC firms dominate high-compliance markets, regional specialists are gaining traction by offering tailored services that cater to the specific needs of local manufacturers. Companies in countries like India, Malaysia, and Thailand have developed a deep understanding of domestic regulatory landscapes, enabling them to provide cost-effective and efficient solutions.
In India, for example, the government's initiatives to boost the medical device sector have led to the growth of local TIC providers. These companies offer services that align with the Indian regulatory framework, assisting domestic manufacturers in achieving compliance and facilitating market access. Their proximity to clients allows for personalized support and quicker turnaround times.
Similarly, in Malaysia and Thailand, regional TIC firms have capitalized on their knowledge of local regulations to offer specialized services. By focusing on niche areas and building strong relationships with domestic manufacturers, these companies have carved out a significant share of the market. Their agility and adaptability make them valuable partners for small and medium-sized enterprises seeking to navigate the complexities of regulatory compliance.
Service Outlook
Based on Service, the market is segmented into Testing, Inspection, and Certification.
Testing Segment
The Testing segment accounts for the largest share of the Asia Pacific medical device TIC outsourcing market. Testing services in this region encompass performance evaluation, biocompatibility, electrical safety, EMC, and sterilization validation. With the rapid expansion of the medical device manufacturing ecosystem in countries like China, India, Japan, and South Korea, stringent regional and international regulations have driven demand for outsourced testing services.
For instance, TÜV SÜD expanded its testing facilities in India and Singapore to accommodate increased volume in Class II and III device submissions, offering in-house support for electrical safety and usability engineering. In 2023, the company collaborated with an Indian orthopedic implant OEM to perform accelerated aging and shelf-life stability testing in compliance with ISO 13485. Similarly, SGS invested in upgrading its Shanghai and Bengaluru labs to offer cytotoxicity and chemical analysis services, helping Chinese and Indian manufacturers navigate MDR and FDA 510(k) requirements more efficiently.
Inspection Segment
The Inspection segment ranks second in terms of market share in the Asia Pacific region. These services include factory audits, in-process quality checks, and pre-shipment inspections. The rise of contract manufacturing and a diversified supply chain landscape across Southeast Asia has made independent third-party inspections critical to ensuring device compliance and production consistency.
Bureau Veritas has expanded its presence in Southeast Asia, particularly in Malaysia and Vietnam, to offer supplier audits and on-site inspection services for orthopedic and cardiovascular device production lines. In 2024, the firm began providing remote inspection support using smart glasses and augmented reality for an Australian respiratory device firm with suppliers in Indonesia and Thailand.
Device Class Outlook
Based on Device Class, the market is segmented into Class II, Class III, and Class I.
Class II Devices Segment
Class II devices constitute the largest share of the Asia Pacific medical device TIC outsourcing market. These devices are generally moderate-risk and include diagnostic imaging systems (e.g., ultrasound, X-ray), infusion pumps, dental surgical instruments, and blood glucose monitors. Because these devices directly interact with patient tissues or bodily functions, stringent conformity assessment procedures, including electrical safety testing, software validation, and biocompatibility evaluation, are essential.
In 2024, Class II devices gained a boost from expanding investments in diagnostic infrastructure across Southeast Asia and India. For instance, in India, Medtronic's expansion of its R&D center in Hyderabad is driving demand for outsourced validation and verification of diagnostic and therapeutic devices. TÜV SÜD and UL Solutions have both reported increased demand for electrical and EMC testing of portable diagnostic devices used in primary healthcare settings.
Class III Devices Segment
Class III devices are high-risk and include implantables such as pacemakers, heart valves, and orthopedic implants. Due to their invasive nature and life-sustaining function, these devices are subjected to exhaustive validation, long-term biocompatibility testing, clinical trials, and post-market surveillance activities.
In 2024, the Class III segment ranked second in market share but accounted for the highest per-unit cost in TIC services due to the complexity of testing protocols. Japanese companies like Terumo and Nipro, which are major manufacturers of cardiac and vascular implants, rely on third-party service providers like SGS and BSI Group for comprehensive product testing and regulatory documentation when exporting to ASEAN and Western markets.
End Use Outlook
Based on End Use, the market is segmented into Medical Device Companies, Pharmaceutical and Biotech Companies, and Other End Use.
Medical Device Companies Segment
Medical device companies held the largest share of the Asia Pacific medical device TIC outsourcing market in 2024, driven by increasing product innovation, stricter international regulations, and rising exports from countries such as China, Japan, India, and South Korea. These companies are outsourcing a wide range of services including product safety testing, electromagnetic compatibility (EMC), software validation, and certification for CE marking and FDA submissions.
The segment is characterized by the need for extensive conformity assessments for a growing portfolio of complex devices such as implants, diagnostic imaging equipment, robotic surgery tools, and wearable monitors. Companies often lack in-house capacity for testing to international standards like ISO 13485, IEC 60601, and IEC 62304, particularly for high-risk and software-integrated devices.
Pharmaceutical and Biotech Companies Segment
Pharmaceutical and biotech companies form a significant end-use category within the TIC outsourcing landscape, especially in the context of combination products and drug-device systems such as pre-filled syringes, autoinjectors, infusion pumps, and inhalers. These products require compliance with both pharmaceutical and medical device standards, prompting companies to outsource rigorous stability, sterility, mechanical, and usability testing.
In 2024, this segment grew due to increasing investments in biologics and biosimilars, where the delivery mechanism is integral to product approval. Many regional biotech startups lack in-house infrastructure for IEC 60601-1 testing, ISO 11608 conformity, or extractables and leachables studies.
List of Key Companies Profiled
- SGS S.A.
- Intertek Group PLC
- Eurofins Scientific SE
- TUV SUD
- ALS Limited
- Bureau Veritas S.A.
- Element Materials Technology (Temasek Holdings)
- DNV AS
- Pace Analytical Services, LLC
- Nelson Laboratories, LLC (Sotera Health Company)
Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market Report Segmentation
By Service
- Testing
- Inspection
- Certification
By Device Class
- Class II
- Class III
- Class I
By End Use
- Medical Device Companies
- Pharmaceutical and Biotech Companies
- Other End Use
By Country
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
1.1 Market Definition
1.2 Objectives
1.3 Market Scope
1.4 Segmentation
1.4.1 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, by Service
1.4.2 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, by Device Class
1.4.3 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, by End Use
1.4.4 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, by Country
1.5 Methodology for the research
Chapter 2. Market at a Glance
2.1 Key Highlights
Chapter 3. Market Overview
3.1 Introduction
3.1.1 Overview
3.2 Key Influencing Factors
3.2.1 Market Drivers
3.2.2 Market Restraints
3.2.3 Market Opportunities
3.2.4 Market Challenges
Chapter 4. Market Trends – Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market
Chapter 5. State of Competition: Medical Device Testing, Inspection And Certification Outsourcing Market
Chapter 6. Competition Analysis – Global
6.1 Market Share Analysis, 2024
6.2 Recent Strategies Deployed in Medical Device Testing, Inspection And Certification Outsourcing Market
6.3 Porter Five Forces Analysis
Chapter 7. Market Consolidation Analysis – Global Medical Device TIC Market
Chapter 8. Product Life Cycle - Global Medical Device TIC Market
Chapter 9. Value Chain Analysis of Medical Device Testing, Inspection And Certification Outsourcing Market
9.1 Market & Regulatory Intelligence
9.2 Test & Inspection Service Provision
9.3 Certification Engagement
9.4 Advisory & Project Management
9.5 Reporting & Data Analytics
9.6 Post-Market Surveillance & Recertification
9.7 Continuous Innovation & Training
Chapter 10. Key Customer Criteria - Medical Device Testing, Inspection And Certification Outsourcing Market
10.1 Regulatory Competence and Global Coverage
10.2 Technical Expertise in Advanced Medical Technologies
10.3 Speed and Flexibility of Service Delivery
10.4 Cost Transparency and Operational Efficiency
10.5 Data Security and Intellectual Property (IP) Protection
10.6 Reputation, Experience, and Post-Market Support
Chapter 11. Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Service
11.1 Asia Pacific Testing Market by Country
11.2 Asia Pacific Inspection Market by Country
11.3 Asia Pacific Certification Market by Country
Chapter 12. Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
12.1 Asia Pacific Class II Market by Country
12.2 Asia Pacific Class III Market by Country
12.3 Asia Pacific Class I Market by Country
Chapter 13. Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
13.1 Asia Pacific Medical Device Companies Market by Country
13.2 Asia Pacific Pharmaceutical and Biotech Companies Market by Country
13.3 Asia Pacific Other End Use Market by Country
Chapter 14. Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Country
14.1 China Medical Device Testing, Inspection And Certification Outsourcing Market
14.1.1 China Medical Device Testing, Inspection And Certification Outsourcing Market by Service
14.1.2 China Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
14.1.3 China Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
14.2 Japan Medical Device Testing, Inspection And Certification Outsourcing Market
14.2.1 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by Service
14.2.2 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
14.2.3 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
14.3 India Medical Device Testing, Inspection And Certification Outsourcing Market
14.3.1 India Medical Device Testing, Inspection And Certification Outsourcing Market by Service
14.3.2 India Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
14.3.3 India Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
14.4 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market
14.4.1 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by Service
14.4.2 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
14.4.3 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
14.4.4 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market
14.4.5 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by Service
14.4.6 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
14.4.7 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
14.5 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market
14.5.1 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by Service
14.5.2 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
14.5.3 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
14.6 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market
14.6.1 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Service
14.6.2 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class
14.6.3 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by End Use
Chapter 15. Company Profiles
15.1 SGS S.A.
15.1.1 Company Overview
15.1.2 Financial Analysis
15.1.3 Segmental and Regional Analysis
15.1.4 Recent strategies and developments:
15.1.4.1 Geographical Expansions:
15.1.5 SWOT Analysis
15.2 Intertek Group PLC
15.2.1 Company Overview
15.2.2 Financial Analysis
15.2.3 Segmental and Regional Analysis
15.2.4 Recent strategies and developments:
15.2.4.1 Partnerships, Collaborations, and Agreements:
15.2.5 SWOT Analysis
15.3 Eurofins Scientific SE
15.3.1 Company Overview
15.3.2 Financial Analysis
15.3.3 Regional Analysis
15.3.4 SWOT Analysis
15.4 TUV SUD
15.4.1 Company Overview
15.4.2 Recent strategies and developments:
15.4.2.1 Geographical Expansions:
15.4.3 SWOT Analysis
15.5 ALS Limited
15.5.1 Company Overview
15.5.2 Financial Analysis
15.5.3 Segmental and Regional Analysis
15.5.4 SWOT Analysis
15.6 Bureau Veritas S.A.
15.6.1 Company Overview
15.6.2 Financial Analysis
15.6.3 Segmental and Regional Analysis
15.6.4 Research & Development Expenses
15.6.5 Recent strategies and developments:
15.6.5.1 Acquisition and Mergers:
15.6.6 SWOT Analysis
15.7 Element Materials Technology (Temasek Holdings)
15.7.1 Company Overview
15.7.2 SWOT Analysis
15.8 DNV AS
15.8.1 Company Overview
15.8.2 Financial Analysis
15.8.3 Segmental and Regional Analysis
15.8.4 SWOT Analysis
15.9 Pace Analytical Services, LLC
15.9.1 Company Overview
15.9.2 SWOT Analysis
15.10. Nelson Laboratories, LLC (Sotera Health Company)
15.10.1 Company Overview
15.10.2 Financial Analysis
15.10.3 Segmental and Regional Analysis
TABLE 2 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 3 Key Customer Criteria: Medical Device Testing, Inspection And Certification Outsourcing Market
TABLE 4 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 5 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 6 Asia Pacific Testing Market by Country, 2021 - 2024, USD Million
TABLE 7 Asia Pacific Testing Market by Country, 2025 - 2032, USD Million
TABLE 8 Asia Pacific Inspection Market by Country, 2021 - 2024, USD Million
TABLE 9 Asia Pacific Inspection Market by Country, 2025 - 2032, USD Million
TABLE 10 Asia Pacific Certification Market by Country, 2021 - 2024, USD Million
TABLE 11 Asia Pacific Certification Market by Country, 2025 - 2032, USD Million
TABLE 12 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 13 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 14 Asia Pacific Class II Market by Country, 2021 - 2024, USD Million
TABLE 15 Asia Pacific Class II Market by Country, 2025 - 2032, USD Million
TABLE 16 Asia Pacific Class III Market by Country, 2021 - 2024, USD Million
TABLE 17 Asia Pacific Class III Market by Country, 2025 - 2032, USD Million
TABLE 18 Asia Pacific Class I Market by Country, 2021 - 2024, USD Million
TABLE 19 Asia Pacific Class I Market by Country, 2025 - 2032, USD Million
TABLE 20 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 21 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 22 Asia Pacific Medical Device Companies Market by Country, 2021 - 2024, USD Million
TABLE 23 Asia Pacific Medical Device Companies Market by Country, 2025 - 2032, USD Million
TABLE 24 Asia Pacific Pharmaceutical and Biotech Companies Market by Country, 2021 - 2024, USD Million
TABLE 25 Asia Pacific Pharmaceutical and Biotech Companies Market by Country, 2025 - 2032, USD Million
TABLE 26 Asia Pacific Other End Use Market by Country, 2021 - 2024, USD Million
TABLE 27 Asia Pacific Other End Use Market by Country, 2025 - 2032, USD Million
TABLE 28 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Country, 2021 - 2024, USD Million
TABLE 29 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Country, 2025 - 2032, USD Million
TABLE 30 China Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2024, USD Million
TABLE 31 China Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 32 China Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 33 China Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 34 China Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 35 China Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 36 China Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 37 China Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 38 Japan Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2024, USD Million
TABLE 39 Japan Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 40 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 41 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 42 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 43 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 44 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 45 Japan Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 46 India Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2024, USD Million
TABLE 47 India Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 48 India Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 49 India Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 50 India Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 51 India Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 52 India Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 53 India Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 54 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2024, USD Million
TABLE 55 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 56 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 57 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 58 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 59 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 60 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 61 South Korea Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 62 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2024, USD Million
TABLE 63 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 64 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 65 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 66 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 67 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 68 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 69 Singapore Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 70 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2024, USD Million
TABLE 71 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 72 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 73 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 74 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 75 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 76 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 77 Malaysia Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 78 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2024, USD Million
TABLE 79 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, 2025 - 2032, USD Million
TABLE 80 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2024, USD Million
TABLE 81 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2025 - 2032, USD Million
TABLE 82 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2024, USD Million
TABLE 83 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2025 - 2032, USD Million
TABLE 84 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2024, USD Million
TABLE 85 Rest of Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2025 - 2032, USD Million
TABLE 86 Key Information – SGS S.A.
TABLE 87 Key Information – Intertek Group PLC
TABLE 88 Key Information – Eurofins Scientific SE
TABLE 89 Key Information – Tuv sud
TABLE 90 Key Information – ALS Limited
TABLE 91 Key Information – Bureau Veritas S.A.
TABLE 92 Key Information – Element Materials Technology
TABLE 93 Key Information – DNV AS
TABLE 94 Key Information – Pace Analytical Services, LLC
TABLE 95 Key Information – Nelson Laboratories, LLC
List of Figures
FIG 1 Methodology for the research
FIG 2 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market, 2021 - 2032, USD Million
FIG 3 Key Factors Impacting Europe Medical Device Testing, Inspection And Certification Outsourcing Market
FIG 4 Market Share Analysis, 2024
FIG 5 Porter’s Five Forces Analysis – Medical Device Testing, Inspection And Certification Outsourcing Market
FIG 6 Market Consolidation Analysis – Global Medical Device TIC Market
FIG 7 Product Life Cycle – Global Medical Device TIC Market
FIG 8 Value Chain Analysis of Medical Device Testing, Inspection And Certification Outsourcing Market
FIG 9 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by Service, 2024
FIG 10 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by Service, 2032
FIG 11 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Service, 2021 - 2032, USD Million
FIG 12 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by Device Class, 2024
FIG 13 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by Device Class, 2032
FIG 14 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Device Class, 2021 - 2032, USD Million
FIG 15 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by End Use, 2024
FIG 16 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by End Use, 2032
FIG 17 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by End Use, 2021 - 2032, USD Million
FIG 18 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by Country, 2024
FIG 19 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market share by Country, 2032
FIG 20 Asia Pacific Medical Device Testing, Inspection And Certification Outsourcing Market by Country, 2025 - 2032, USD Million
FIG 21 Swot Analysis: SGS S.A.
FIG 22 Swot Analysis: Intertek Group PLC
FIG 23 SWOT Analysis: Eurofins Scientific SE
FIG 24 Swot Analysis: TUV SUD
FIG 25 Swot Analysis: ALS LIMITED
FIG 26 Swot Analysis: Bureau Veritas S.A.
FIG 27 SWOT Analysis: Element Materials Technology
FIG 28 Swot Analysis: DNV AS
FIG 29 Swot Analysis: Pace Analytical Services, LLC